Enthusiasm for the use of open-label placebos – or placebos without deception or concealment – has increased in recent years. Many factors have contributed to this increase in popularity, including debate around whether traditional placebo techniques (which typically involve some form of deception) can be used ethically in clinical practice. Several studies have demonstrated the effectiveness of open-label placebos in irritable bowel syndrome, depression, and itch; however, the mechanisms underlying open-label placebo analgesia are unknown.
Now, researchers led by Fabrizio Benedetti, University of Turin Medical School, Italy, have added another layer to the placebo phenomenon, reporting that in an experimental setting, naloxone also blocks open-label placebo-induced analgesia.
“There has been a need for more research into how open-label placebo works, as we don’t have a consensus mechanistic explanation for how these effects occur,” said Luana Colloca, director of the Placebo Beyond Opinions Center at University of Maryland School of Nursing, Baltimore, US. Colloca has extensive knowledge in the neurobiological mechanisms of placebo and nocebo effects but was not involved in the current research.
“This paper adds a missing piece to our understanding of the neurobiology underlying open-label placebo analgesia – that there is a release of endogenous opioids that can be blocked by naloxone.”
The research was published in PAIN on 20 September 2022.
Blocking the effects of open-label placebo analgesia: The final frontier?
Placebo analgesia is a well-known phenomenon in the pain field. This effect is thought to be triggered by expectations resulting from verbal suggestions, conditioning, social observations, and interpersonal interactions, which promote the release of endogenous opioids. Subsequent research has shown administration of naloxone, an opioid antagonist, prevents placebo analgesia from occurring.
While placebo analgesia has its benefits, it is not without its challenges. Many clinicians and researchers have discussed the ethics of using placebo in research and in the clinic, particularly in relation to the use of deception.
This led to the identification of open-label placebos, where the deception aspect is removed. That is, the placebo is given with verbal instructions that it is a fake or inert treatment. Several randomized trials of open-label placebo have demonstrated open-label placebos to be effective.
The apparent effectiveness of open-label placebos raises the possibility that simply the act of taking a pill or applying a cream – even if you know it is a placebo – can elicit the placebo effect, as the ritual induces positive expectations of improvement and/or conditioned responses by the mere act of self-administration. Consequently, these earlier studies led researchers to wonder if open-label placebo analgesia activates the same endogenous opioid mechanisms as traditional placebo analgesia.
“Non-deceptive placebos challenge the concept that placebo effects need deception,” lead author Benedetti told PRF via email. “For a neuroscientist like me, one of the main questions is to understand whether this difference implies different [neurobiological] mechanisms.”
So Benedetti and colleagues designed a study to assess whether non-deceptive open-label placebo analgesia could be antagonized by blocking opioid receptors with naloxone.
Putting the theory to the test
The authors recruited 149 pain-free participants, but only 69 participants (including 29 women) were exposed to the open-label placebo aspect of the study. Experimental pain was induced via the tourniquet technique. In brief, participants raised their left arm and venous blood was drained. A blood pressure cuff was applied and inflated before the arm was lowered. Participants were instructed to squeeze a hand exerciser, producing an ischemic pain that increases over time.
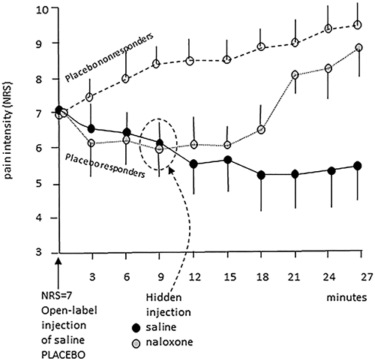
When participants in the experimental group rated the ischemic pain as a 7 out of 10, they received an injection of saline via an intravenous line that had been placed in their right arm at the beginning of the session. The intravenous line ran into an adjacent room, where the experimenters could administer injections without participants knowing. All participants could see and were told that they were receiving saline, and the experimenter explained why open-label placebos might be effective. A particular emphasis was placed on the notion that the ritual of receiving an injection may be enough to trigger an analgesic response.
Participants provided pain ratings at three-minute intervals following the saline injection. If participants rated their pain as a 7 or less after 9 minutes, they were deemed to be a placebo responder. Participants who rated their pain as an 8 or above were deemed placebo non-responders. Twenty-eight participants (41%) were placebo responders, with the remaining 41 (59%) being placebo non-responders.
The placebo responders received a “hidden” injection (by an experimenter in the adjacent room) at this point in the experiment. The placebo responders were randomized to receive either a second injection of saline (14 participants) or a 10 mg dose of naloxone (14 participants). Placebo non-responders did not receive a second injection. All participants continued to provide pain ratings for an additional 18 minutes.
Unsurprisingly, the placebo non-responders reported increases in pain intensity for the remainder of the experiment. The “hidden” saline injection administered to the placebo responders did not affect placebo analgesia; participants reported decreases in pain intensity. However, participants who received the “hidden” naloxone injection reported increased pain intensity (mean pain intensity rating at 27 minutes = 8.8), implying the opioid antagonist had successfully blocked the open-label placebo analgesia.
How does this change the way we think about placebos?
The observation that naloxone blocked open-label placebo analgesia suggests that deception is not a requirement to activate the endogenous opioid system. For Benedetti, this reinforces the notion that the act, or ritual, of administering a drug may be sufficient to release opioids in the brain – even if we know the drug is fake.
Benedetti draws parallels between this proposed mechanism and the psychophysiological response to other situations that we know are fake, such as movies.
“Horror movies induce psychophysiological responses even though we know everything is fake. Indeed, we should expect fear, heartbeat increase, sweating, goosebumps, etc., only in real circumstances – when survival is at stake. Nonetheless, we have these physiological reactions when watching a movie,” he explained.
It is important not to get too caught up, however, as the new research does have some limitations. These findings are a prime candidate for replication and further study due to the small sample sizes, the arbitrary basis by which placebo responders were identified, and the potential clinical translatability of the ischemic pain model compared to other approaches.
Despite these limitations, Colloca feels these findings sit well among the broader field of placebo research.
“I hope [this study] will incite curiosity and enthusiasm around endogenous pain modulation to see how we can harness the power of the inner pharmacy and the release of endogenous opioids to help patients cope with their pain.”
Lincoln Tracy is a researcher and freelance writer from Melbourne, Australia. You can follow him on Twitter – @lincolntracy.
References
Benedetti F, Shaibani A, Arduino C, Thoen W. Open-label nondeceptive placebo analgesia is blocked by the opioid antagonist naloxone. PAIN. 2022. doi:10.1097/j.pain.0000000000002791. PMID: 36165878
Featured Image
Figure 4 – Benedetti et al. Placebo non-responders always scored more than 7 after 9 minutes from the open-label placebo. Conversely, placebo responders never rated pain more than 7 at this time interval. Placebo responders were further subdivided in those receiving hidden saline or naloxone at 9 minutes. Note that naloxone antagonized placebo analgesia whereas saline did not. Vertical lines are standard deviations. NRS, numerical rating scale.