Fostering discussion and collaboration that speeds up the acquisition of new knowledge and its translation into novel pain treatments.
PRF News
Papers Of The Week
The gut microbiota promotes pain in fibromyalgia.
Thalamic CGRP neurons define a spinothalamic pathway for affective pain.
Pain is both a sensory and emotional experience caused by various harmful stimuli. While numerous studies have explored peripheral and central pain mechanisms, the specific neural circuits linking the spinal cord to the brain remain poorly defined. In this study, we demonstrate the involvement of calcitonin gene-related peptide (CGRP)-positive neurons in the parvicellular part of the subparafascicular nucleus (SPFp) in pain. Tracing revealed that CGRP neurons in the SPFp (CGRP) receive projections from the dorsal horn. Increased calcium activity was observed in CGRP neurons during mechanical, thermal, and inflammatory stimuli. Genetic silencing of these neurons resulted in reduced pain responses in animals. Furthermore, optogenetic activation of CGRP neurons induced aversive memory but did not alter mechanical or thermal pain thresholds. This study reveals a distinct neural circuit involving CGRP neurons that mediates pain, which differs from CGRP neurons in the parabrachial nucleus. Understanding these circuits could lead to better pain treatments with fewer side effects.
Opposite regulation of medullary pain-related projection neuron excitability in acute and chronic pain
Pain hypersensitivity is associated with increased activity of peripheral and central neurons along the pain neuroaxis. We show that at the peak of acute inflammatory pain, superficial medullary dorsal horn projection neurons (PNs) that relay nociceptive information to the parabrachial nucleus reduce their intrinsic excitability and, consequently, action potential firing. When pain resolves, the excitability of these neurons returns to baseline. Using electrophysiological and computational approaches, we found that an increase in potassium A-current (IA) underlies the decrease in the excitability of medullary dorsal horn PNs in acute pain conditions. In chronic pain conditions, no changes of IA were observed, and medullary dorsal horn PNs exhibit increased intrinsic excitability and firing. Our results reveal a differential modulation of the excitability of medullary dorsal horn projection neurons in acute and chronic pain conditions, suggesting a regulatory mechanism that, in acute pain conditions, tunes the output of the dorsal horn and, if lacking, could facilitate pain chronification.
Enhancer AAVs for targeting spinal motor neurons and descending motor pathways in rodents and macaque
Experimental access to cell types within the mammalian spinal cord is severely limited by the availability of genetic tools. To enable access to spinal motor neurons (SMNs) and SMN subtypes, we generated single-cell multiome datasets from mouse and macaque spinal cords and discovered putative enhancers for each neuronal population. We cloned these enhancers into adeno-associated viral vectors driving a reporter fluorophore and functionally screened them in the mouse. We extensively characterized the most promising candidate enhancers in rat and macaque and developed an optimized pan-SMN enhancer virus. Additionally, we generated derivative viruses expressing iCre297T recombinase or ChR2-EYFP for labeling and functional studies, and we created a single vector with combined enhancer elements to achieve simultaneous labeling of layer 5 extratelencephalic projecting neurons and SMNs. This unprecedented SMN toolkit will enable future investigations of cell type function across species and potential therapeutic interventions for human neurodegenerative diseases.
Rejuvenation alleviates prolonged postsurgical pain in aging mice by mitigating inflammaging.
As individuals age, they often experience persistent, unresolved pain, impacting their quality of life. Aging as a process is accompanied by “inflammaging,” a state of chronic, low-grade systemic inflammation contributing to various diseases. Understanding the functional link between inflammaging and age-related development of pain is crucial for identifying novel therapeutic targets. We hypothesized that the circulatory milieu plays a role in regulating pain and that inflammaging contributes to changes in pain behavior with age. To test these hypotheses, we monitored nociception and postsurgical pain in male and female mice aged 3 and 24 months and analyzed their serum proteome, including cytokine/chemokine profiles. Our results demonstrated that compared with young mice, aging mice were hyposensitive to mechanical stimulation, yet their pain response to incision was aggravated and prolonged. Serum proteomic analysis revealed sex-specific inflammaging patterns. To explore the link between inflammaging and age-related alteration in pain behavior, we applied a rejuvenation strategy by transferring serum from 3-month-old mice to 19- to 21-month-old mice. Young serum normalized mechanical sensitivity in aged mice, alleviated postsurgical mechanical pain, and promoted recovery. Alongside the improvements in pain behavior phenotype, young serum recalibrated the aging serum profile. It reduced age-associated increases of cytokine/chemokine levels in male mice and rescued age-related, female-selective downregulation of inflammatory pathways such as liver X receptor/retinoid X receptor activation, D24-dehydrocholesterol reductase, and complement signaling. Our findings suggest that the circulatory environment, notably inflammaging, plays a significant role in altered pain behavior of aging mice. The sex-specific signature of age-dependent systemic inflammation highlights the importance of investigating inflammaging through the lens of sexual dimorphism.
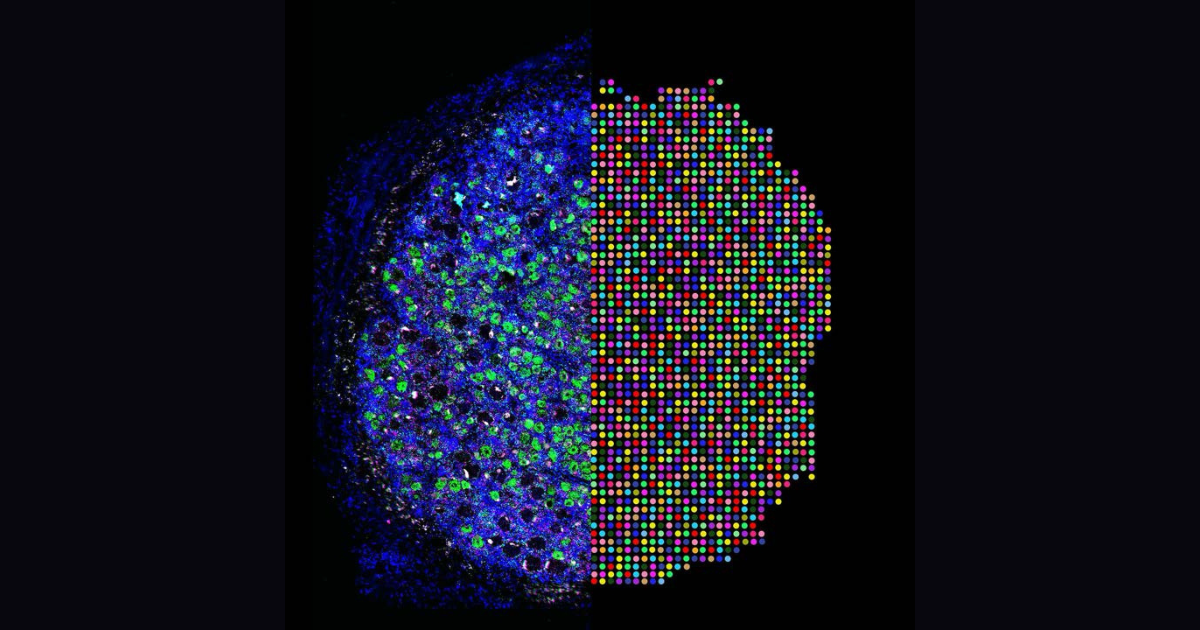
Projection-TAGs enable multiplex projection tracing and multi-modal profiling of projection neurons
Single-cell multiomic techniques have sparked immense interest in developing a comprehensive multi-modal map of diverse neuronal cell types and their brain-wide projections. However, investigating the complex wiring diagram, spatial organization, transcriptional, and epigenetic landscapes of brain-wide projection neurons is hampered by the lack of efficient and easily adoptable tools. Here we introduce Projection-TAGs, a retrograde AAV platform that allows multiplex tagging of projection neurons using RNA barcodes. By using Projection-TAGs, we performed multiplex projection tracing of the cortex and high-throughput single-cell profiling of the transcriptional and epigenetic landscapes of the cortical projection neurons in female mice. Projection-TAGs can be leveraged to obtain a snapshot of activity-dependent recruitment of distinct projection neurons and their molecular features in the context of a specific stimulus. Given its flexibility, usability, and compatibility, we envision that Projection-TAGs can be readily applied to build a comprehensive multi-modal map of brain neuronal cell types and their projections.
Discovery of a functionally selective serotonin receptor (5-HTR) agonist for the treatment of pain.
The heterotrimeric G protein-coupled serotonin receptor 5-HT receptor (5-HTR) mediates antinociception and may serve as a valuable target for the treatment of pain. Starting from a chemical library, we evolved ST171, a bitopic 5-HTR agonist that revealed highly potent and functionally selective G signaling without G activation and marginal β-arrestin recruitment. ST171 is effective in acute and chronic pain models. Cryo-electron microscopy structures of ST171 bound to 5-HTR in complex with the G protein compared to the canonical agonist befiradol bound to complexes of 5-HTR with G or G revealed that the ligands occupy different exo-sites. The individual binding poses are associated with ligand-specific receptor conformations that were further studied by molecular dynamics simulations, allowing us to better understand ligand bias, a phenomenon that may be crucial to the discovery of more effective and safe G protein-coupled receptor drugs.
Microglial pruning of glycinergic synapses disinhibits spinal PKCγ interneurons to drive pain hypersensitivity in mice.
Microglial activation is linked to neuroinflammation in neuropathic pain. Recently, microglia-mediated synaptic pruning has received mounting attention. However, the exact role of spinal microglia in modulating neuropathic pain-associated neural circuits remains unclear. To investigate this question, we used pharmacological, optogenetic, and genetic manipulations combined with behavioral tests, confocal imaging, and patch-clamp studies in a murine spared nerve injury (SNI) model of neuropathic pain. We demonstrate that spinal microglia pruned inhibitory presynaptic terminals in SNI mice, contributing to the disinhibition of spinal protein kinase C γ (PKCγ) interneurons and facilitating neurotransmission from low-threshold Aβ fibers. Single-cell RNA sequencing revealed that SNI-associated microglial subpopulations exhibited high expression of liver X receptor, apolipoprotein E (), and complement C1q. Global knockout of , microglia-specific knockdown of , or treatment with anti-C1q monoclonal antibody reversed SNI-induced pruning of spinal inhibitory synapses, prevented the disinhibition of PKCγ interneurons, and reduced pain hypersensitivity. Our study suggests that destabilization of neural networks through microglia-mediated pruning of inhibitory synapses in the spinal cord contributes to the development of neuropathic pain in mice.
About PRF
IASP's Pain Research Forum serves as a nucleus for scientists performing basic, translational, and clinical pain
research, as well as clinicians and industry stakeholders interested in advances in pain research and
management.
About PRF
The interactive web community dedicated to accelerating the discovery of new treatments for pain.
PRF Job Listings
Find information about opportunities in academia, industry, and more.
PRF Editorial Board
We're guided by an international Editorial Board of leading pain researchers.
Contact PRF
PRF welcomes any comments, questions, suggestions, or potential submissions you may have.
IASP Job Board
Find information on interviewing, networking, and even more opportunities.