Fostering discussion and collaboration that speeds up the acquisition of new knowledge and its translation into novel pain treatments.
PRF News
Papers Of The Week
Enhancer AAVs for targeting spinal motor neurons and descending motor pathways in rodents and macaque
Discovery of a functionally selective serotonin receptor (5-HTR) agonist for the treatment of pain.
The heterotrimeric G protein-coupled serotonin receptor 5-HT receptor (5-HTR) mediates antinociception and may serve as a valuable target for the treatment of pain. Starting from a chemical library, we evolved ST171, a bitopic 5-HTR agonist that revealed highly potent and functionally selective G signaling without G activation and marginal β-arrestin recruitment. ST171 is effective in acute and chronic pain models. Cryo-electron microscopy structures of ST171 bound to 5-HTR in complex with the G protein compared to the canonical agonist befiradol bound to complexes of 5-HTR with G or G revealed that the ligands occupy different exo-sites. The individual binding poses are associated with ligand-specific receptor conformations that were further studied by molecular dynamics simulations, allowing us to better understand ligand bias, a phenomenon that may be crucial to the discovery of more effective and safe G protein-coupled receptor drugs.
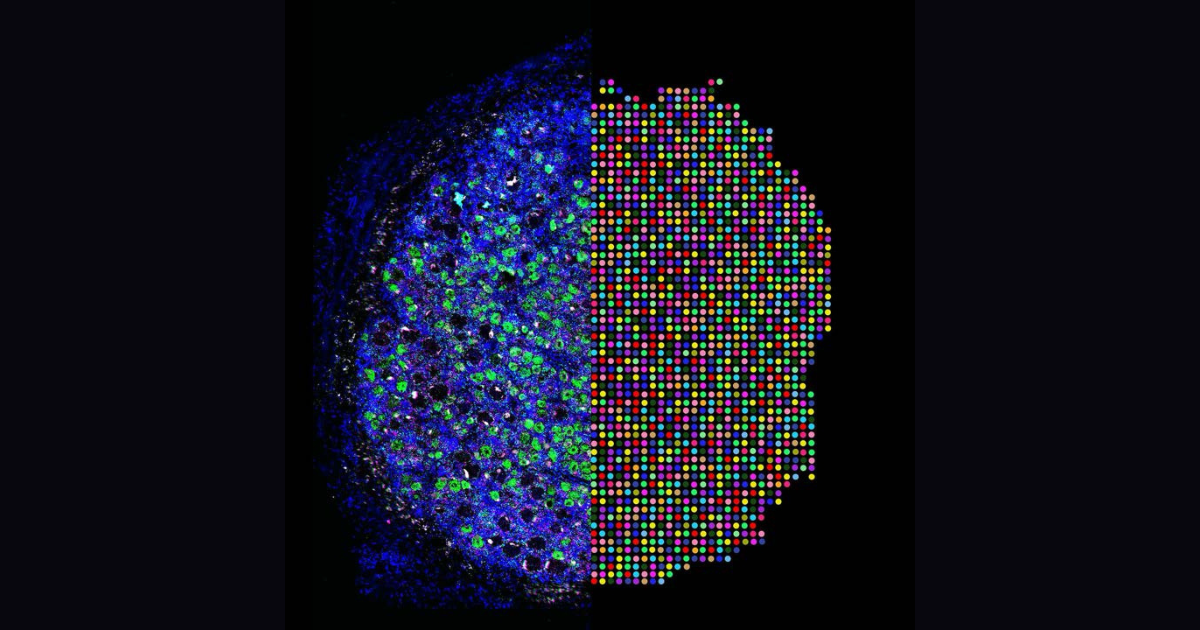
Microglial pruning of glycinergic synapses disinhibits spinal PKCγ interneurons to drive pain hypersensitivity in mice.
Microglial activation is linked to neuroinflammation in neuropathic pain. Recently, microglia-mediated synaptic pruning has received mounting attention. However, the exact role of spinal microglia in modulating neuropathic pain-associated neural circuits remains unclear. To investigate this question, we used pharmacological, optogenetic, and genetic manipulations combined with behavioral tests, confocal imaging, and patch-clamp studies in a murine spared nerve injury (SNI) model of neuropathic pain. We demonstrate that spinal microglia pruned inhibitory presynaptic terminals in SNI mice, contributing to the disinhibition of spinal protein kinase C γ (PKCγ) interneurons and facilitating neurotransmission from low-threshold Aβ fibers. Single-cell RNA sequencing revealed that SNI-associated microglial subpopulations exhibited high expression of liver X receptor, apolipoprotein E (), and complement C1q. Global knockout of , microglia-specific knockdown of , or treatment with anti-C1q monoclonal antibody reversed SNI-induced pruning of spinal inhibitory synapses, prevented the disinhibition of PKCγ interneurons, and reduced pain hypersensitivity. Our study suggests that destabilization of neural networks through microglia-mediated pruning of inhibitory synapses in the spinal cord contributes to the development of neuropathic pain in mice.
Environmental microbiomes drive chemotactile sensation in octopus
Microbial communities coat nearly every surface in the environment and have co-existed with animals throughout evolution. Whether animals exploit omnipresent microbial cues to navigate their surroundings is not well understood. Octopuses use “taste-by-touch” chemotactile receptors (CRs) to explore the seafloor, but how they distinguish meaningful surfaces from the rocks and crevices they encounter is unknown. Here, we report that secreted signals from microbiomes of ecologically relevant surfaces activate CRs to guide octopus behavior. Distinct molecules isolated from individual bacterial strains located on prey or eggs bind single CRs in subtly different structural conformations to elicit specific mechanisms of receptor activation, ion permeation and signal transduction, and maternal care and predation behavior. Thus, microbiomes on ecological surfaces act at the level of primary sensory receptors to inform behavior. Our study demonstrates that uncovering interkingdom interactions is essential to understanding how animal sensory systems evolved in a microbe-rich world.
Lamellar Schwann cells in the Pacinian corpuscle potentiate vibration perception
Pacinian corpuscles are among the most sensitive mechanoreceptors found in vertebrates, and they are tuned to vibrations in the highest perceptible frequency range (100 to 2000 Hz). One of their anatomical hallmarks is the onion-like cell layers surrounding the central axon. The innermost layers consist of ~60 densely packed lamellar Schwann cells (LSCs), whose function remains largely unknown. Using high-resolution three-dimensional electron microscopy, we found that LSCs do not form concentric rings, but complex, multilayered, and intertwining assemblies that are connected via a high density of desmosomes and gap junctions. LSCs make multiple converging contacts with the afferent axon via desmosomes. Using optogenetic manipulations of LSCs, we demonstrate not only that their activation drives reliable time-locked spiking in the axon but also that their inactivation significantly elevates the thresholds in situ and increases perceptual thresholds behaviorally. Together, these findings provide evidence that LSCs are a key element of somatosensory processing, actively potentiating mechanosensitivity in Pacinian corpuscles.
PACAP and migraine.
A number of neuropeptides including pituitary adenylate cyclase-activating polypeptide (PACAP) play an important role in the pathophysiology of migraine. Infusions of PACAP in patients with migraine can provoked migraine attacks. A placebo-controlled study with a monoclonal antibody directed against the PACAP-receptor failed to show efficacy. In a small, short, proof of concept study a monoclonal antibody directed against PACAP (Lu AG09222) showed efficacy in the reduction of monthly migraine days compared to placebo, but failed for the endpoint 50%-reduction in migraine days. The ongoing PROCEED-study is a double-blind, placebo-controlled, dose-finding study investigating four different doses of Lu AG09222 vs placebo for migraine prevention is expected to complete in the second half of 2025.
Sex differences in the transition to chronic pain.
Chronic pain affects more than 50 million Americans, with women disproportionately affected by severe pain, pain interference, and overall disability. The development of chronic pain is multifactorial and often begins with an incident of acute pain associated with an injury or a surgical procedure that transitions to persistent pain lasting for months or years. Despite this, there are limited clinical studies investigating sex differences in predictors and biomarkers for the transition to chronic pain. Several preclinical animal models have been developed to gain a better understanding of the mechanisms for the transition to chronic pain, and several sex-specific mechanisms have been identified across multiple systems. These preclinical models generally involve a multiple-insult approach, in which a priming insult enhances sensitivity to a subsequent induction stimulus. There is emerging evidence from preclinical research for several male-specific and female-specific mechanisms, as well as several studies showing shared mechanisms. Here, we review the clinical and preclinical literature covering sex differences in the periphery and immune system, the central nervous system, and the endocrine system related to the transition to chronic pain. We further highlight gaps in the literature and provide recommendations for future research to understand sex-specific differences in the transition to chronic pain.
Mechanism-based nonopioid analgesic targets.
Acute pain management has historically been dominated by opioids, whose efficacy is overshadowed by the risks of addiction, tolerance, and dependence, culminating in the global opioid crisis. To transcend this issue, we must innovate beyond opioid-based μ receptor treatments, identifying nonopioid analgesics with high efficacy and minimal adverse effects. This Review navigates the multifaceted landscape of inflammatory, neuropathic, and nociplastic pain, emphasizing mechanism-based analgesic targets tailored to specific pain conditions. We delve into the challenges and breakthroughs in clinical trials targeting ion channels, GPCRs, and other molecular targets. We also highlight the intricate crosstalk between different physiological systems and the need for multimodal interventions with distinct pharmacodynamics to manage acute and chronic pain, respectively. Furthermore, we explore emerging strategies, including gene therapy, stem cell therapy, cell type-specific neuromodulation, and AI-driven techniques for objective, unbiased pain assessment and research. These innovative approaches are poised to revolutionize pain management, paving the way for the discovery of safer and more effective analgesics.
About PRF
IASP's Pain Research Forum serves as a nucleus for scientists performing basic, translational, and clinical pain
research, as well as clinicians and industry stakeholders interested in advances in pain research and
management.
About PRF
The interactive web community dedicated to accelerating the discovery of new treatments for pain.
PRF Job Listings
Find information about opportunities in academia, industry, and more.
PRF Editorial Board
We're guided by an international Editorial Board of leading pain researchers.
Contact PRF
PRF welcomes any comments, questions, suggestions, or potential submissions you may have.
IASP Job Board
Find information on interviewing, networking, and even more opportunities.