Pain research has created a wealth of knowledge about pain and pain management, yet much of that knowledge has not been communicated to the public or made an impact on clinical practice in a meaningful way. Some of these challenges can be overcome by partnering with people with lived experience of pain along the knowledge translation spectrum – including in research, education, practice (see Global Year Fact Sheet on Clinical Practice Guidelines), and policymaking.
People with lived experience bring more than just their pain experiences to these projects, they also bring their lifetime of education, work, life experiences and their unique talents, skills, and creativity. Partnering with people with lived experience helps ensure the relevance of research, improve the quality and delivery of health care services for people living with pain, and improves patient and public health outcomes, all of which lead to more meaningful and wider-reaching impacts[2,3,24,25]. Plus, including people with lived experience in pain in research is just the right thing to do[1].
Terminology
Many terms are used to describe people with lived experience partnering in research and within healthcare systems[24], including patient and/or public involvement, patient engagement, public participation, or patient partnerships. In some regions, other terms may be used in place of patient or public, such as stakeholder, consumer, citizen, and/or community. Such research collaborations may also be referred to as participatory [action] research, citizen science, patient-oriented research, or community- or practice-based research networks.
The process of partnering with people with lived experience in research is referred to in a variety of ways, including co-creation[14], co-production[8], co-design[16], and co-development. Such collaborative practices may also be reflected in initiatives around inclusivity, diversity, representation, health equity, and social justice[10].
‘Co-producing a research project is an approach in which researchers, practitioners and the public work together, sharing power and responsibility from the start to the end of the project, including the generation of knowledge[12].’ (https://www.learningforinvolvement.org.uk)
In co-produced research, people with lived experience are often referred to as ‘patient partners’ (can also refer to carers, family members, and patient advocates), yet they are not patients in these roles. Rather, people with lived experience are members of the research team who bring their lived expertise, knowledge, and insights to inform the design, execution, and mobilization of projects.
How to Include People with Lived Experience with Pain in Research
There is a rich and growing practice of involving people with lived experience in research, and many research funders now require meaningful patient engagement in the research process[21]. People with lived experience can be involved throughout the research process[15], including setting priorities; establishing research agendas; applying for funding; developing research questions; designing and overseeing of studies[7]; recruiting participants; collecting, analyzing and interpreting data; co-authoring and publishing peer-reviewed papers[19]; and sharing research findings to patients, clinicians, and the general public. An interactive roadmap that includes information and resources at each stage can be found here: A Journey Through Public & Patient Engagement in Health Research: A Road Map.
The International Association for Public Participation (IAP2) has identified five levels of engagement with each level along the continuum reflecting greater patient and public involvement. The stages are: inform, consult, involve, collaborate, and empower:
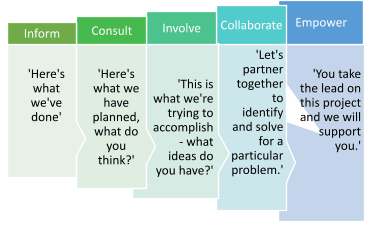
People with lived experience of pain can be involved at all levels of the translational science spectrum as well, including in basic, preclinical[9], and clinical research[22]; clinical implementation; and public health initiatives[6]. There are a number of frameworks and resources available, including, NIHR Training and Resources for Public Involvement in Research website[13]; the Strategy for Patient-Oriented Research – Patient Engagement Framework from Canadian Institutes of Health Research[5]; and this How-to Guide for Patient Engagement in the Early Discovery and Preclinical Phases[18] from Patient Focused Medicines Development.
How to Involve People with Lived Experience in Knowledge Mobilization and Dissemination
People with lived experience can also help share and mobilize the knowledge about pain that we have gained through pain research in recent decades. They can co-produce public information campaigns, programs in schools and communities, plain language summaries of current and existing research, and help share this knowledge through videos, social media, popular media formats, blogs/vlogs, infographics, and other means of communicating information. Co-design and delivery of such materials can increase relevance, accessibility, representation, and practicality – how to put knowledge into action for clinicians, patients and the public. (For more, see the Translating Knowledge into Practice Fact Sheet).
Best Practices for Research Engagement
Equity, diversity, inclusion, and representation
To have the greatest impact, it is important to ensure the people with lived experience of the conditions being studied are included. It is also important that all members of the communities for whom services are being co-designed are represented, including those from racialized, minoritized, marginalized, and historically excluded populations[4,16,17]. (See Addressing Inequities fact sheet for more on this topic.)
Recognition and compensation
It is important to recognize and value the time, expertise, and contributions that people with lived experience provide21. Reimbursement or upfront payment of expenses patient partners incur as a result of their contributions should always be included. It is also best practice to offer compensation. Other ways to recognize and value the contributions of patient partners include acknowledgements in research articles and other public forums, co-authorship on manuscripts, invitations to speak at conferences or in classrooms (with expenses paid), and access to knowledge, training, and resources[23]. Here is a handy guide on talking with patient partners about compensation[20].
How to Evaluate It
Evaluation approaches should also be co-created with research partners who have lived experience[15]. There are a number of approaches to evaluation, which can be qualitative or quantitative[15]. There are also multiple themes that can be evaluated, such as the structure of how people with lived experience were involved in the project, the processes employed, or the outcomes of various stages of the project[11].
PUBLICLY AVAILABLE ONLINE RESOURCES
- National Institute for Health Research (NIHR) Learning for involvement
- A Journey through Public & Patient Engagement in Health Research: A Road Map
- Canadian Institutes of Health Research (CIHR): Strategy for Patient-Oriented Research – Patient Engagement Framework
- International Association for Public Participation
- Ontario SPOR Support Unit Patient Engagement Resources
REVIEWERS
Dawn Richards PhD
Ian Graham MA PhD
Katherine Boydell MHSc PhD
REFERENCES
[1] Belton J, Hoens A, Scott A, Ardern CL. Patients as Partners in Research: It’s the Right Thing to Do. J Orthop Sports Phys Ther 2019;49:623–626.
[2] Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, Suleman R. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Patient 2014;7:387–395.
[3] Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, Suleman R. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expectations 2014;17:637–650.
[4] Buchman DZ, Ho A, Goldberg DS. Investigating Trust, Expertise, and Epistemic Injustice in Chronic Pain. J Bioeth Inq 2017;14:31–42.
[5] Canadian Institutes of Health Research. Strategy for Patient-Oriented Research: Patient Engagement Framework. Ottawa, ON: Canadian Institutes of Health Research, 2019 Available: http://www.cihr-irsc.gc.ca/e/48413.html.
[6] Collaboration and Partnerships. National Center for Advancing Translational Sciences 2015. Available: https://ncats.nih.gov/translation/issues/partner. Accessed 15 Dec 2021.
[7] Coulman KD, Nicholson A, Shaw A, Daykin A, Selman LE, Macefield R, Shorter GW, Cramer H, Sydes MR, Gamble C, Pick ME, Taylor G, Lane JA. Understanding and optimising patient and public involvement in trial oversight: an ethnographic study of eight clinical trials. Trials 2020;21:543.
[8] COVID-19 and Co-production in Health and Social Care Research, Policy, and Practice. 1st ed. Bristol University Press, 2021 Available: http://www.jstor.org/stable/j.ctv1p6hqk9. Accessed 17 Dec 2021.
[9] Fox G, Fergusson DA, Daham Z, Youssef M, Foster M, Poole E, Sharif A, Richards DP, Hendrick K, Mendelson AA, Macala KF, Monfaredi Z, Montroy J, Fiest KM, Presseau J, Lalu MM. Patient engagement in preclinical laboratory research: A scoping review. EBioMedicine 2021;70:103484.
[10] Goldberg DS. On Stigma & Health. J Law Med Ethics 2017;45:475–483.
[11] Hamilton CB, Dehnadi M, Snow ME, Clark N, Lui M, McLean J, Mamdani H, Kooijman AL, Bubber V, Hoefer T, Patients as Partners Team, Li LC. Themes for evaluating the quality of initiatives to engage patients and family caregivers in decision-making in healthcare systems: a scoping review. BMJ Open 2021;11:e050208.
[12] INVOLVE Advisory Group. Guidance on co-producing a research project. 2018. Available: https://www.invo.org.uk/wp-content/uploads/2019/04/Copro_Guidance_Feb19.pdf. Accessed 15 Dec 2021.
[13] Involve patients | NIHR. n.d. Available: https://www.nihr.ac.uk/health-and-care-professionals/engagement-and-participation-in-research/involve-patients.htm. Accessed 15 Dec 2021.
[14] Kaisler RE, Missbach B. Co-creating a patient and public involvement and engagement ‘how to’ guide for researchers. Res Involv Engagem 2020;6:32.
[15] Martinez J, Wong C, Piersol CV, Bieber DC, Perry BL, Leland NE. Stakeholder engagement in research: a scoping review of current evaluation methods. J Comp Eff Res 2019;8:1327–1341.
[16] Moll S, Wyndham-West M, Mulvale G, Park S, Buettgen A, Phoenix M, Fleisig R, Bruce E. Are you really doing ‘codesign’? Critical reflections when working with vulnerable populations. BMJ Open 2020;10:e038339.
[17] Ocloo J, Garfield S, Franklin BD, Dawson S. Exploring the theory, barriers and enablers for patient and public involvement across health, social care and patient safety: a systematic review of reviews. Health Res Policy Sys 2021;19:8.
[18] Patient Focused Medicines Development. How-to guide for patient engagement in the early discovery and preclinical phases. 2020. Available: https://pemsuite.org/How-to-Guides/Early-Discovery.pdf. Accessed 15 Dec 2021.
[19] Richards DP, Jordan I, Strain K, Press Z. Patient partner compensation in research and health care: the patient perspective on why and how. Patient Experience Journal 2018;5:6–12.
[20] Richards DP, Jordan I, Strain K, Press Z. Patients as Partners in Research: How to Talk About Compensation With Patient Partners. Journal of Orthopaedic & Sports Physical Therapy 2020;50:413–414.
[21] Russell J, Fudge N, Greenhalgh T. The impact of public involvement in health research: what are we measuring? Why are we measuring it? Should we stop measuring it? Res Involv Engagem 2020;6:63.
[22] Selman LE, Clement C, Douglas M, Douglas K, Taylor J, Metcalfe C, Lane JA, Horwood J. Patient and public involvement in randomised clinical trials: a mixed-methods study of a clinical trials unit to identify good practice, barriers and facilitators. Trials 2021;22:735.
[23] Smith E, Bélisle-Pipon J-C, Resnik D. Patients as Research Partners; How to Value their Perceptions, Contribution and Labor? Citizen Science: Theory and Practice 2019;4:15.
[24] Tembo D, Hickey G, Montenegro C, Chandler D, Nelson E, Porter K, Dikomitis L, Chambers M, Chimbari M, Mumba N, Beresford P, Ekiikina PO, Musesengwa R, Staniszewska S, Coldham T, Rennard U. Effective engagement and involvement with community stakeholders in the co-production of global health research. BMJ 2021:n178.
[25] Vat LE, Finlay T, Jan Schuitmaker‐Warnaar T, Fahy N, Robinson P, Boudes M, Diaz A, Ferrer E, Hivert V, Purman G, Kürzinger M, Kroes RA, Hey C, Broerse JEW. Evaluating the “return on patient engagement initiatives” in medicines research and development: A literature review. Health Expectations 2020;23:5–18.



