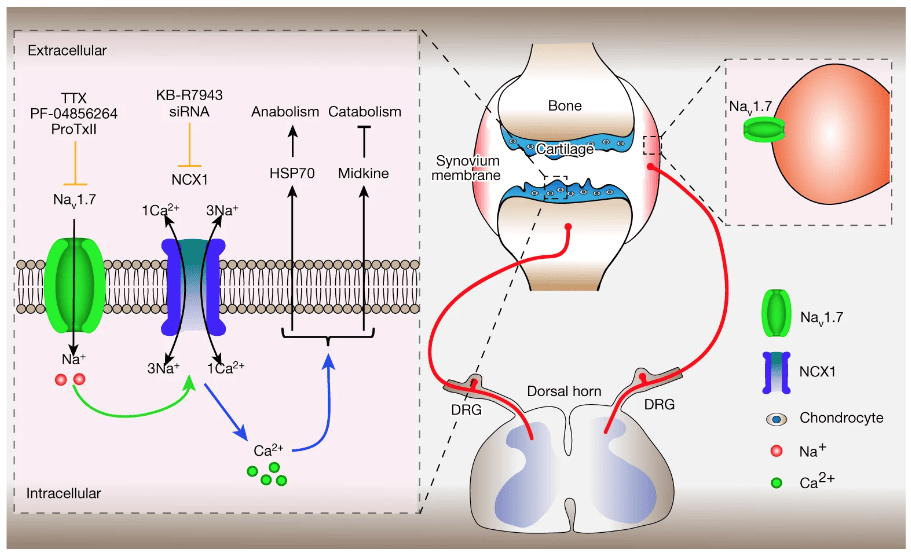
Osteoarthritis is a debilitating disorder involving cartilage breakdown, joint degeneration, and pain. There are currently no disease-modifying treatments for osteoarthritis. Chondrocytes – the cells responsible for cartilage formation – are known to play a key role in osteoarthritis development, but there are many unknowns about the specific molecular mechanisms involved in this condition, including the potential role of voltage-gated sodium channels.
Now, research led by Wenyu Fu and Dmytro Vasylyev (Yale School of Medicine, Connecticut, USA) has demonstrated that human chondrocytes express functional Nav1.7 channels which contribute to osteoarthritis progression and pain-related behaviors.
Professor Ewan St. John Smith (Department of Pharmacology, University of Cambridge, UK) described the findings as an important step in understanding more about disease pathogenesis and pain in osteoarthritis.
“The fact they’ve demonstrated a functional role for Nav1.7 in chondrocytes for osteoarthritis pathogenesis and pain is quite exciting, because it opens up the potential to modify either the channel in the chondrocytes or to perhaps target those mediators they identify as HSP70 [involved in the quality control of newly synthesized proteins] and midkine [a growth factor that inhibits the breakdown of large molecules] as potential disease-modifying pathways,” he told Pain Research Forum.
“We know that pain is the main driver of clinical decision-making, [but] if you stop the pathogenesis, you perhaps don’t need to worry about the pain. This isn’t just showing another target for pain, but this is a target [involved] with pain because of the pathogenesis.”
The research and an accompanying commentary from the authors were published in Nature on 3 January 2024.
“An exciting convergence”
Senior authors Stephen Waxman and Chuan-Ji Liu (both from the Yale School of Medicine, USA) have known each other for a long time. Liu worked in Waxman’s lab more than 20 years ago, which served as something of a nucleus for studying Nav1.7.
“During the 1990s we showed sodium channels were present in low densities in a number of non-excitable neuron types – astrocytes, oligodendrocytes, fibroblasts, microglia, and macrophages. At that time, we did really elegant electrophysiology, pharmacology, and biophysics, but the story didn’t get any traction because it was all in vitro, and we didn’t have a function for these channels,” Waxman told Pain Research Forum.
Previous work in many labs had found sodium channels were expressed in a range of typically “excitable” cells, including neurons, cardiomyocytes, and skeletal muscle cells.
However, Liu found something unexpected: Six different voltage-gated sodium channels were expressed in human chondrocytes – and that Nav1.7 expression was increased in chondrocytes isolated from people with osteoarthritis. What’s more, Nav1.7 expression increased proportionally to osteoarthritis progression.
“I was surprised when we found [voltage-gated] sodium channels in the non-excitable chondrocytes, [and] thought it could be a very interesting discovery. Once we confirmed the expression of Nav1.7, I reached out to Steve [Waxman] to set up further electrophysiology experiments,” Liu recalled.
“It was an exciting convergence of two labs,” Waxman said of his reunion with Liu. “Collaborations are driven by mutual interest, trust, and a common approach – but also by complementary expertise.”
This complementary expertise included joint first authors Fu and Vasylyev, who made substantial contributions to the in vivo and electrophysiology work, respectively.
Nav1.7: More than just a bit player in osteoarthritis
After proving Nav1.7 channels were expressed in a small proportion of human chondrocytes (albeit at a very low density of ~0.1-0.15 channels/μm2, or ~350-525 channels per cell) and were associated with osteoarthritis, the next step was to figure out what role the channels played, and whether the channels expressed on chondrocytes functioned similarly to those on dorsal root ganglia (DRG).
To investigate, the researchers generated three groups of knockout mice: One missing Nav1.7 channels in the DRG neurons, one missing Nav1.7 channels in the chondrocytes, and one missing Nav1.7 in both the DRG neurons and the chondrocytes.
The researchers used surgical and chemical approaches to model disease progression and osteoarthritis-related pain in the three groups of knockout mice.
Removing Nav1.7 channels from the DRG neurons reduced open field movement activity and mechanical allodynia in the surgical model of osteoarthritis without affecting the structure of the joints.
Notably, the removal of Nav1.7 from the chondrocytes reduced cartilage loss, osteophyte formation, subchondral bone plate thickening, and synovitis score, and decreased the subsequent osteoarthritis-associated pain behaviors in the surgical model.
The dual knockout mouse model displayed reduced osteoarthritis progression and pain-related behaviors in both the surgical and chemical osteoarthritis models.
St. John Smith said the dual role for Nav1.7-expressing chondrocytes in osteoarthritis was a key finding.
“It demonstrates Nav1.7 [expressed] in cartilage is playing a functional role regardless of what its expression level is. Demonstrating that the ion channel contributes to disease pathogenesis due to its function in a non-neuronal cell type is an important result,” he told Pain Research Forum.
Further experiments with the selective Nav1.7 blocker PF-04856264 and the clinically used sodium channel blocker carbamazepine showed these treatments were protective against cartilage loss and pain in the osteoarthritis mouse models.
“These findings suggest that non-specific voltage-gated sodium channel [blockers] – such as carbamazepine – may hold promise as novel disease-modifying treatments for osteoarthritis, and do not simply act in an analgesic manner,” the authors wrote.
Some answers, more questions
Blocking Nav1.7 with PF-04856264 and carbamazepine also upregulated HSP70 and midkine, two proteins previously implicated in chondrocyte biology, in cell and animal models.
However, inhibiting HSP70 and midkine reduced the protective effects seen by blocking Nav1.7. This highlights the importance of these proteins in maintaining the protective effects of Nav1.7 inhibition in osteoarthritis – and shows that the Nav1.7 blockade partly influences chondrocyte anabolism and catabolism through regulating HSP70 and midkine secretion.
Measuring intracellular ion concentrations suggested the cascade of activity initiated by blocking Nav1.7 involved calcium signaling via the NCX family of proteins (also called solute carrier family 8 proteins), as preventing NCX from regulating calcium signaling stopped the secretion of HSP70 and midkine caused by Nav1.7 inhibition.
“At the distal end of the causal pathway, we know Nav1.7 allows a sodium influx that drives the sodium-calcium exchange to operate in reverse, which elevates calcium levels. In turn, that modulates the secretome of these cells – there’s a multiplicative component,” Waxman said.
It’s not entirely clear to Waxman, Liu, or St. John Smith how such a small number of Nav1.7 channels can have such a profound effect on chondrocyte activity, cartilage damage, and pain.
“We’re taking a close look at this right now, and I hope we’ll be able to explain it in a year or so,” Waxman explained.
Lincoln Tracy is a researcher and freelance writer from Melbourne, Australia. You can follow him on X – @lincolntracy.
Featured image:
Figure 4m: Fu et al. Nature. 3 January 2024; 625:557-565. Model of mechanisms of chondrocyte- and DRG-expressed Nav1.7 in osteoarthritis, and amelioration of osteoarthritis and pain via Nav1.7 blockade.
References:
Fu W, Vasylyev D, Bi Y et al. Nav1.7 as a chondrocyte regulator and therapeutic target for osteoarthritis. Nature 625, 557–565 (2024). doi: 10.1038/s41586-023-06888-7
Waxman SG and Liu C. Small numbers of sodium channels on cartilage cells have a large effect on joint damage. Nature (2024). doi: 10.1038/d41586-023-03845-2