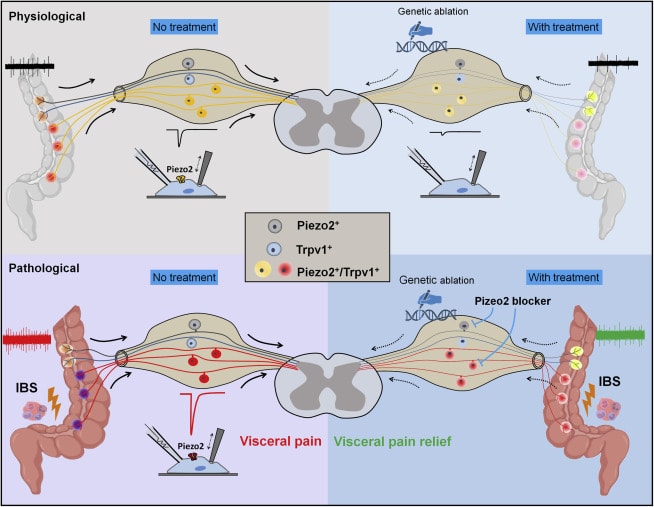
Visceral pain is a common complaint among patients with functional gastrointestinal disorders such as irritable bowel syndrome (IBS) and functional dyspepsia. It is also highly comorbid with stress-related conditions such as depression, anxiety, and fatigue. Despite its prevalence, the underlying mechanisms responsible for visceral pain remain poorly understood. Furthermore, visceral pain has received far less attention – in terms of research and drug development – compared to somatic pain, despite being a more prominent clinical issue.
Recent research led by Zili Xie, Washington University School of Medicine, Missouri, USA, has identified a significant contributor to visceral pain: Mechanosensitive Piezo2 channels expressed by extrinsic TRPV1-lineage nociceptors that innervate the gut.
Associate Professor Arthur Beyder, The Mayo Clinic, Minnesota, USA, felt the findings were a significant step forward in the understanding of how visceral pain works.
“The biggest barrier for us has been finding the ‘light switch’ that turns visceral pain on. The wiring and light bulbs that come after that are important, but if the light bulb came on, the problem isn’t the light bulb – the problem is the ‘switch’ that turned it on.
“Identifying the role of Piezo channels gives us the true opportunity – for the first time – to determine where the ‘light switches’ are and how they work with respect to the generation and propagation of visceral pain,” he told Pain Research Forum.
The research was published in the 15 February 2023 edition of Neuron, and an accompanying commentary can be found here.
Confirming the role of TRPV1 in visceral mechanical pain
A variety of noxious stimuli – chemical, thermal, and mechanical – can activate somatosensory neurons. In contrast, mechanical stimulation – such as distension or mesenteric traction – is the primary form of painful stimulation in visceral organs, including the gastrointestinal tract. Researchers have focused on understanding the role of sensory ion channels in mechanotransduction and mechanical hypersensitivity.
Despite the known emphasis on mechanical forces, much of the knowledge about visceral pain derives from experiments focusing on somatic pain. Our skin and joints are far more accessible – and therefore more easily studied – compared to visceral organs. Consequently, there are only a small number of animal models for visceral pain compared to other, well-established inflammatory and neuropathic pain models for somatic pain research.
The gastrointestinal tract expresses a variety of ion channels in a diverse range of cell types, including voltage-gated calcium and sodium ion channels, as well as transient receptor potential (TRP) channels. Two such channels – TRPV1 and TRPA1 – have previously been shown to play a role in visceral mechanotransduction and visceral pain, and recent research has identified Piezo1 and Piezo2 channels as legitimate mechanically gated ion channels (Coste et al., 2010; Murthy et al., 2018; Szczot et al., 2018; Hill et al., 2022). However, little is known about the role of Piezo ion channels in visceral mechanotransduction and visceral pain.
Xie and colleagues aimed to explore the role of Piezo ion channels in visceral mechanical signaling. They first characterized colonic spinal sensory neurons in a specifically cross-bred mouse model and used retrograde tracing to reveal that almost all colon-innervating primary sensory neurons originating from the thoracolumbar and lumbosacral dorsal root ganglia were TRPV1-expressing neurons. This initial finding suggests TRPV1-expressing sensory afferents play an important role in visceral mechanical transduction, as well as hypersensitivity in the mouse colon.
The authors then chemically ablated all TRPV1-expressing sensory neurons in the colon using resiniferatoxin – an ultrapotent agonist for TRPV1 receptors. Compared to vehicle-treated mice, those that underwent chemical ablation had significantly reduced colorectal distention-induced visceromotor responses. More selective ablation of colon-innervating TRPV1-expressing sensory neurons using an adeno-associated virus containing a diphtheria toxin subunit also resulted in significantly reduced colorectal distention-induced visceromotor responses, providing compelling evidence that the TRPV1-expressing colon-innervating neurons make a significant contribution to mechanical transduction in the mouse colon.
Turning our attention to Piezo
After observing a role of TRPV1 neurons in mechanical transduction, Xie and colleagues then sought to determine if and how Piezo2 contributed to visceral mechanosensitivity in the gastrointestinal tract. The authors used single-cell quantitative reverse transcriptase PCR analysis on lumbosacral dorsal root ganglion neurons to show extensive overlap between Piezo2 and Trpv1 mRNA transcripts, implying that the TRPV1-expressing visceral nociceptors may also express Piezo2 channels.
To explore this further, the authors then generated nociceptor-specific Piezo2 knockout mice, where Piezo2 channel function was selectively ablated from TRPV1-expressing nociceptors. Ex vivo afferent recordings from colonic preparations revealed significantly reduced circumferential stretch-evoked action potential firing in the knockout mice compared to wild-type littermates. Mechanical probing and mucosal brushing of the colonic preparations showed the same decreased response.
The knockout mice also displayed significantly attenuated colorectal distension-induced visceromotor responses across three different pressures, demonstrating the Piezo2 channels expressed on TRPV1 neurons are sensitive to different types of mechanical stimuli and mediate colonic afferent firing.
Joint senior author Hongzhen Hu was somewhat surprised by the results.
“This finding suggests that the Piezo2 channel plays a major role in the generation of visceral but not somatic mechanical pain under physiological conditions. We speculate that this might explain why the visceral sensory system is primarily activated by distension and/or stretch,” he told PRF.
Mouse models of gastrointestinal disorders
The authors used zymosan, a macromolecule derived from the cell wall of yeast, to replicate the visceral hypersensitivity seen in IBS without affecting the structure or inflammatory profile of the colon. The zymosan treatment significantly increased the expression of Piezo2 in dorsal root ganglion neurons, which in turn increased the colorectal distension visceromotor responses compared to controls. Taken together, these data indicate that the contribution of Piezo2 to visceral mechanotransduction is increased by zymosan treatment.
Xie and colleagues also explored the role of Piezo2 on visceral mechanical hypersensitivity in a mouse model of partial colon obstruction. There were no significant differences in the inflammatory response following a partial colonic obstruction between the general Piezo2 and Piezo2 TRPV1-specific knockout mouse models, suggesting Piezo2 does not contribute to the inflammatory response produced by the obstruction. However, there was a significant increase in Piezo2 channel expression in lumbosacral dorsal root ganglion neurons following partial colonic obstruction, like that observed in the zymosan IBS mouse model.
There were also decreases in visceromotor responses following colonic distension in the Piezo2 TRPV1-specific knockout mouse model post-colonic obstruction. This suggests the Piezo2 channels associated with TRPV1 neurons make significant contributions to the partial colon obstruction-enhanced visceral mechanical hypersensitivity.
The changes in Piezo channel function in the two clinical models caught the attention of Tijs Louwies, a postdoctoral researcher at The Mayo Clinic with expertise in the epigenetic regulation of visceral hypersensitivity.
“The data seem to suggest that more neurons start to express Piezo2 after zymosan or partial colonic obstruction, rather than the Piezo2-expressing afferents significantly increasing their basal expression,” he told PRF.
Finally, voluntary movements in an open field test were not affected in the zymosan-treatment mice but were significantly reduced following partial colonic obstruction – particularly in the Piezo2 knockout mouse model. These conflicting results suggest that while zymosan-induced IBS hypersensitivity is insufficient to produce spontaneous visceral pain, Piezo2 makes an important contribution to the generation of spontaneous visceral pain in the partial colonic obstruction mouse model.
Part of a bigger picture
When considering the findings from this current study with existing research demonstrating a role for TRPV1 in visceral hypersensitivity, Hu speculates that “TRPV1-lineage neurons regulate chemosensory responses through sensitization of TRPA1/TRPV1 channels and regulate mechanosensory mechanisms via sensitization of Piezo2.”
However, Hu notes that as previous research suggests voltage-gated calcium channels also contribute to the pathogenesis of visceral pain, “the interaction and integration of functions of various ion channels shape the duration and intensity of visceral mechanical nociception and hypersensitivity.”
Beyder agrees that Piezo2 and TRPV1 may only explain part of the story.
“This was a natural question to ask. I’m glad they did it, and glad they got the answers they did, but it’s very clearly not the only thing going on. In every way they’ve looked at the data, ablated receptors, or approached it from different angles, pain signaling has only been reduced by 30% to 40%.”
Beyder suggested that mucosal and epithelial factors may also contribute to visceral mechanical hypersensitivity.
Hu and his team plan to continue to identify inflammatory signaling pathways involved in Piezo2-mediated visceral mechanical hypersensitivity in the setting of gastrointestinal inflammation and obstruction.
“We believe these unique signaling pathways can be targeted to treat visceral pain.”
Lincoln Tracy is a researcher and freelance writer from Melbourne, Australia. You can follow him on Twitter – @lincolntracy.
Featured image: Graphical abstract. Xu, J, McGinnis, A, and Ji, R. Piezo2 mediates visceral mechanosensation: A new therapeutic target for gut pain? Neuron. 15 Feb 2023.