Thirteen early-career pain researchers and clinicians are taking part in the third cycle of the PRF Virtual Correspondents Program. This science communications training program provides participants with knowledge and skills needed to communicate science effectively to a wide range of pain researchers, and to patients and the broader public. Throughout the course of the program, the Correspondents will conduct interviews and podcasts with leading pain researchers, provide news and virtual meeting coverage – and blog posts! Take a look at their posts below, which will be published weekly over the next six weeks.
Meet the PRF Correspondents

Week 6: Wednesday, April 14, 2021
A Multidisciplinary and Holistic Approach to Pain Management
Pain: Not Only a Neuronal Affair
Words Matter When Talking About Pain
Words Matter When Prescribing Exercises
Tweets Containing #pain or #migraine: What Do People Write About?
Working With, Instead of Fighting Against, the Chronic Pain Monster
Cold Pain in the Teeth Reimagined: A Key Role for TRPC5 in Odontoblasts
The Link Between Fibromyalgia, Women, and Autoimmunity
Precision Medicine: Diagnosing and Treating Central Sensitization
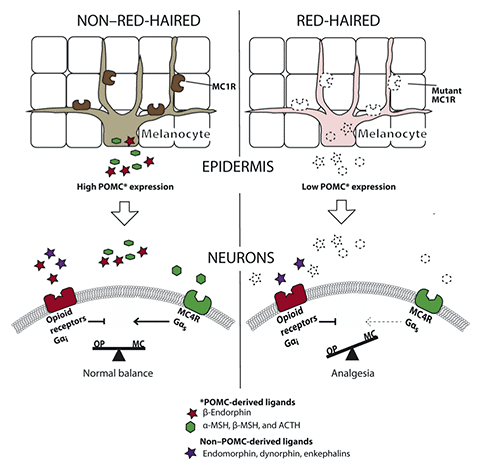
Remember Anne of Green Gables? That’s the name I would give to one of the mice in this study if I were the author. In this recent investigation, red-haired mice (wait, what?) are used to explore the mechanism that underlies the low sensitivity to acute painful stimuli observed in redheads.
But let’s take a step back first: What causes red hair? Genetic variants in the melanocortin 1 receptor (MC1R) expressed on melanocytes – the pigment-producing cells of the skin – are responsible for red hair. MC1R is a G protein-coupled receptor that, once activated by melanocyte-stimulating hormone (MSH), leads to the production of the brown/black pigment eumelanin. In people with loss-of-function variants, to which MSH cannot bind, the pigment pheomelanin (yellow/red) is produced instead. It should be noted that aside from MC1R, other genes contribute to red hair, and variants in MC1R are often also observed in blonde individuals.
The question arises whether loss-of-function alterations in MC1R are also responsible for changes in pain sensitivity. In a cohort of 17 redheads (just imagine an Irish football team) it was found that higher pain thresholds were associated with regulatory variants (mutations in untranslated regions), rather than missense mutations. From the prevalence of different variants in previous studies and their own, the authors estimated that about half of redheads will have a “pain-protective” phenotype.
Back to our mouse Anne. The researchers wanted to understand the mechanism that mediates these high pain thresholds observed in red-haired individuals. To do so, they used a mouse strain with a defective MC1R, to recapitulate the human loss-of-function variants. These mice showed increased pain thresholds to thermal and mechanical stimuli, as previously reported. This effect of MC1R was determined by its expression in melanocytes, but independent from pigment production.
The red-haired mice were secreting lower levels of proopiomelanocortin, a precursor molecule of MSH and the endogenous opioid β-endorphin. While the latter is antinociceptive through activation of opioid receptors, MSH is pronociceptive. In line with this, administration of an MSH-mimicking peptide to red-haired mice matched their pain thresholds to those of black mice. This pointed the authors towards a melanocortin receptor other than MC1R as responsible for MSH actions. Using knockout mice, MC4R was identified as such.
Finally, based on co-localization experiments, the authors propose that MC4R antagonizes opioid receptors (see illustration below). Therefore, in red-haired mice, the low levels of MSH result in reduced antagonism of opioid receptors and ultimately low pain sensitivity. The flipside to this pain resistance is the sensitivity to ultraviolet (UV) rays and skin cancer risk. Which one would you prefer if you could choose?
Larissa de Clauser, PhD from University College London, now based in Italy.
Before delving into my final post, I would like to say a massive thank you to PRF and RELIEF for the opportunity to participate in this program, and to you, the PRF community, for your engagement with the blog. I had a great time writing these posts and I hope you have enjoyed reading them!
Last year I woke up in the middle of the night to a relentless pain emanating from my right big toe. It was so bad that even the weight of the sheet was unbearable. Looking down I saw that my toe was red, swollen and hot to the touch. Had I stubbed it? Had I dropped something on it? Well, no, unfortunately I knew it was likely something else because, from what I could see, it looked quite a lot like gout.
Not a great realization, I have to admit. Most people, including myself, associate gout – a form of inflammatory arthritis – with somewhat of an excessive lifestyle; this is something that has not been helped by its previous and ill-advised nickname, the “disease of kings. ”As someone who is relatively young and healthy (plus a pescatarian and a woman!), I couldn’t quite understand why I would suddenly develop it.
Of course, the first thing I did was go online to do some research and while doing so I realized that gout could in fact be a possibility. You see, my father has fallen victim to its attacks for many decades, and with gout having a high level of heritability, he may seemingly have passed on the joys of this fate to me (thanks Dad!). Alongside the finding that the typical Western diet is associated with a 42% increased risk of developing gout, I suppose it is less surprising a prospect than I had previously thought.
Gout is a systemic disease, occurring when hyperuricemia – a sustained increase in serum urate levels resulting in oversaturation of urate in body tissue – leads to the formation and deposition of monosodium urate crystals in and around the joints. These crystals initiate inflammatory processes by being engulfed by macrophages, the body’s phagocytic immune cells, which in turn leads to the release of pro-inflammatory cytokines. Recurrent gout can cause major disability via chronic inflammation and joint degradation, with sufferers’ main outward symptom being pain. Luckily though, there is a definitive curative treatment for gout via therapies that lower urate levels.
Through reading I discovered that gout is actually the most common form of inflammatory arthritis globally, with a high prevalence in developed countries along with a worrying rise in case numbers. It is more prevalent in men than women, and in the older population, and has an array of predisposing risk factors including obesity and diabetes, along with the previously mentioned genetic susceptibility. A major contributor to gout is of course diet, with consumptions of purine-rich foods, such as red meat and seafood, leading to increased uric acid precursors and gout onset.
Unsurprisingly, as a long-term sufferer my father is well versed in what to avoid food-wise, so while I was on the phone telling him of my recent misfortune, he provided some advice: "Definitely avoid hoppy beer. And be careful of red wine. Oh, and pulses…. Seafood can also do it." Not ideal, as he was essentially listing the foodstuffs I enjoy.
Thankfully, the symptoms of my attack diminished within a day. Without an opportunity for a diagnosis at that time I am still reluctant to admit that it was actually gout. Only the next flare will tell, and in the meantime, no beer nor lentil dhals for me!
Oakley Morgan, PhD student, University College London, UK.
A Multidisciplinary and Holistic Approach to Pain Management
“Not only is it important to ask questions and find the answers, as a scientist I felt obligated to communicate with the world what we were learning.” – Stephen Hawking
The PRF Correspondents Program epitomizes leadership in providing the tools for effective science communication. Being a part of this program has allowed me to engage with the broader PRF and IASP community while also providing an opportunity to communicate my research.
I had anticipated that this program would help me refine my writing skills but what I did not factor in was the cathartic process of writing itself. With every blog I re-visited memories of my medical and research training, the amazing people I have met along the way, and the plethora of experiences I've had.
I explored five constructs in my blog posts: the power of social support and relationships; dyadic coping (intimate partner-provided support); moving from treating pain to caring for the patient; overcoming language barriers in order to provide equal healthcare opportunities for all; and our collective role in meeting the challenge of healthcare disparities.
I chose to write about these specific themes because they illustrate the need for a multidisciplinary and holistic approach to pain management; the impact of pain on almost all facets of life, including physical health, sleep, mental health, relationships, social life and employment makes this approach the best path forward. It will help improve pain disability, insomnia, and psychological distress in our patients while helping them reconnect with activities that bring joy and meaning to their life.
I am enjoying every minute of this program and I want to especially thank our PRF editor, Neil Andrews, for his encouragement and unwavering support. I also applaud my fellow Correspondents for sharing their own stories and making this process fun and exciting.
Finally, I want to thank you, our readers, for your encouragement, comments and thoughts. It’s been an absolute pleasure.
I am continuing my efforts to communicate science with an exciting upcoming podcast with Professor Judith Turner. Stay tuned…
Follow me on Twitter @DrManasiMurthy
Manasi M. Mittinty, MD, PhD, lecturer, University of Sydney, Australia.
Pain: Not Only a Neuronal Affair
When we think about pain, whether as a scientist, clinician or patient, there is a general understanding that it is a neurobiological response involving the brain and neurons. As researchers we understand that there is detection of noxious stimuli by nociceptors, processing in the brain and movement responses. However, pain does not rely entirely on neurons; other cells are also involved. In particular, immune cells are involved at multiple stages of pain processing, including in the spinal cord, and these cells generally release inflammatory mediators to enhance nociceptive signaling.
In the skin, the ends of nociceptive neurons have historically been described as “free.” That is, they extend out almost like branches of a tree with no leaves, detecting noxious stimuli. This was always noteworthy as the neurons in the skin responsible for other sensations like touch, vibration and pressure have specialized organs surrounding them that help to transmit the signal from the skin to the neurons – more like branches of a tree that have fruit on the end. Nociceptive neurons were described as free as they did not have a specialized structure that served a similar purpose.
However, recent work has indicated that there are nociceptive neurons whose endings are not free and are surrounded by a mesh of non-neuronal cells that interact with the neurons. These cells were dubbed nociceptive Schwann cells. The research group behind this work, led by Patrik Ernfors, used light to determine whether the network of Schwann cells has any effect on pain. The technique is called optogenetics and involves genetically inserting a light-sensitive protein into neurons in order to activate or turn off the cells upon light exposure. When the light was turned on and aimed at the paw of mice to activate only the Schwann cells, the mice flinched and retracted their paws as if they were in pain. This suggested that the Schwann cells do interact with neurons and are involved in nociceptive signaling.
The question remained as to whether these cells are involved in all types of pain or a specific type. To answer this, the group used optogenetics and saw that the cells were inherently and primarily mechanosensitive, though there was some involvement with cold and heat pain. This is particularly interesting as our understanding of the way that neurons detect mechanical pain has been quite elusive, perhaps in part because the neurons require help from other sources.
For researchers interested in this debate, could this provide more evidence for polymodal nociceptors (nociceptive neurons that respond to a variety of noxious stimuli)? Perhaps neurons that respond to hot or cold can also respond to mechanical pain with the help of the mechanically-sensitive Schwann cells. It is a possible avenue of further study.
Excitingly, not only can non-neuronal cells also detect mechanical stimuli and transmit that to neurons, but it may eventually be time to change the textbooks about the long-held view of nociceptive neurons as having free nerve endings in the skin.
Frederick Jones, CASE PhD student, University of Leeds, UK, and Eli Lilly & Co, US.
Words Matter When Talking About Pain
Words are important. The language we use and the advice and guidance we share has great significance to a person living with pain. The words we use may carry a sense of hope and possibility or be associated with pessimism and low expectations. In fact, the words used in clinical conversations and in writing can influence a person’s mood, self-esteem, feelings of happiness or depression, understanding of their problem, and can even influence pain itself.
A growing body of research has shown that the words clinicians use to describe a health condition can also influence a person’s decisions about treatment. For example, words that elicit strong negative reactions may make you more likely to choose surgical treatment even when equally effective and safer treatment options are available.
A recent study found that the clinician’s use of certain medical words might be encouraging people with shoulder pain to say “yes” to unnecessary shoulder surgery. This randomized controlled trial investigated the use of six diagnostic labels for a common type of shoulder pain. Participants were presented with six hypothetical scenarios with the only difference between scenarios being the words used to describe the person’s shoulder pain. People told they had a “rotator cuff tear” wanted surgery the most whereas people told they had “bursitis” (inflammation of a fluid-filled sac in the shoulder) wanted surgery the least.
Given how important words can be to a person in pain, it is imperative that clinicians consider whether the words they use to describe a condition might be causing unnecessary fear or concern. Changing the words we use and the stories we tell to describe painful conditions is a simple strategy that could improve the lives of people living with pain.
Aidan G. Cashin, PhD Candidate, Neuroscience Research Australia (NeuRA); University of New South Wales.
Words Matter When Prescribing Exercises
Exercise is a core component of the management of chronic pain conditions. Many different types of exercise help reduce pain and disability, and have general positive effects on cardiovascular health, mental health and sleep, among others.
One of the many reasons why exercise is beneficial for pain is the exercise-induced hypoalgesia phenomenon. Studies have shown that even a single session of exercise can have a positive effect on pain sensitivity and pain tolerance. This means that doing aerobic or resistance exercise directly reduces pain, like taking a pill. While this phenomenon is very consistent in pain-free individuals, the positive effects are not as strong in people with chronic pain.
A recent study investigated if information given before exercise can influence the hypoalgesia effect of exercise. Researchers randomized people into positive (“studies have shown that exercise reduce pain”), neutral (only information about how to perform the exercise) and negative (“studies have shown that exercise can induce pain”) pre-exercise information groups. The study revealed that, while the positive and neutral information groups showed a hypoalgesia effect with exercise, the negative information group showed the opposite – an increase in pain sensitivity (hyperalgesia effect).
How do these results relate to the prescription of exercise for people with pain? Many studies have shown that explanations from health professionals may have a strong biomechanical orientation. For instance, frequent advice for low back pain is that people should be active, but they should also be careful to not injure their back. They receive encouragement to exercise but at the same time they are told to control their posture, to activate their deep muscles, to move in the “right” way or to be careful not to do too much. This conflicting advice may increase the perceived threat of exercise and have a negative effect with regard to exercise-induced hypoalgesia. Additionally, it can also negatively impact self-efficacy and result in kinesiophobia or unhelpful beliefs.
Therefore, to enhance the positive effect of exercise in people with chronic pain, clinicians should promote positive messages about the effect of exercise. They should also discuss potential unhelpful beliefs about doing exercise, and promote self-efficacy and confidence.
Words matter – and probably even more than the type of exercise, when it comes to pain.
Guillaume Christe, PhD student, Haute École de Santé Vaud (HESAV), Switzerland.
Tweets Containing #pain or #migraine: What Do People Write About?
Many people these days spend countless of hours on social media writing about everything between heaven and earth. As a health and medical scientist involved in pain research, I am interested in what people write about pain and migraine and how they do it. Therefore, I decided to conduct a preliminary sentiment analysis of Twitter content to find out. Sentiment has been described as “a metric commonly used to investigate the positive or negative opinion” within tweets. So it can tell us something about the tone of tweets.
Unsurprisingly, negative sentiments are used more often in tweets containing #pain or #migraine than positive sentiments are. So, what negative sentiments are most commonly used? I looked into the top 20 negative sentiments in tweets posted between April 2 to April 9, 2021. There were 11 negative sentiments that the tweets containing #pain or #migraine had in common. Of these sentiments, four caught my attention: stress, anxiety, depression, and inflammation. I decided to further dissect those using information from the literature.
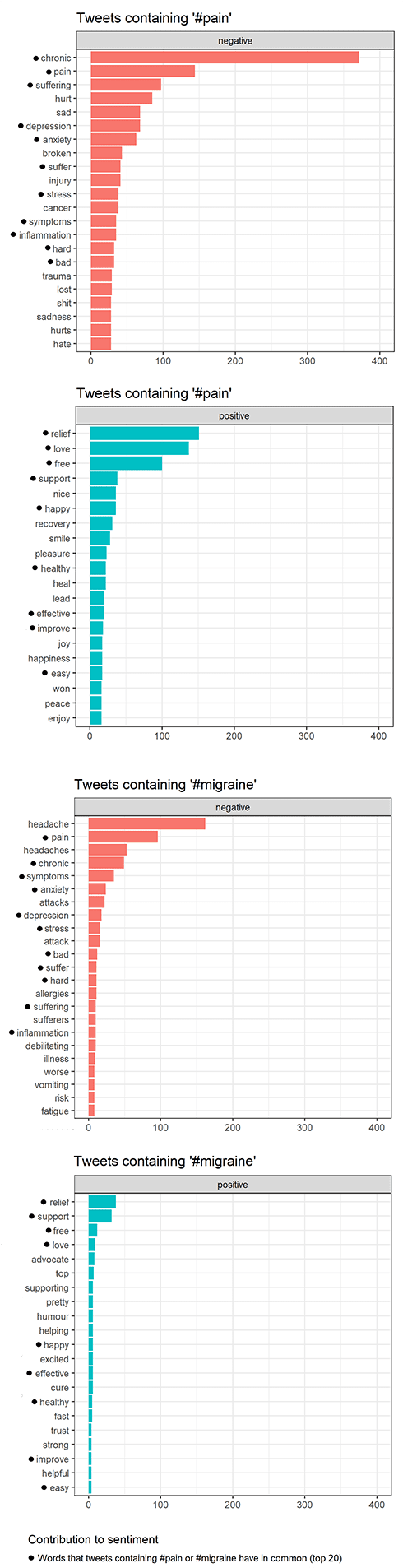
The sentiment analysis was conducted using Twitter content available through the Twitter API and is therefore subject to the terms and conditions put forward by Twitter. Credit: The R package rtweet by Kearney MW (2019) was used for collecting tweets and carrying out the sentiment analysis. Thanks to Vajiha Sipra for inspiration.
Stress
The link between stress and pain is well-known. However, which comes first depends on the situation. We know that pain can cause stress and that stress can cause pain. Stress is also known to be a trigger of migraine attacks and is widely recognized as an important factor in disease.
Anxiety and depression
Anxiety and chronic pain tend to co-occur (see here and here). Interestingly, anxiety is a larger contributor to the risk of developing migraine than depression. Nevertheless, headache frequency tends to increase if patients are experiencing both anxiety and depression. There also seems to be a connection between the severity of anxiety and living with migraine.
Inflammation
In the central nervous system, altered proinflammatory molecule profiles have been observed in the context of chronic pain, with increased pain sensitivity and pain arising from a stimulus that would not normally be considered painful. Controlling inflammation in the nervous system has been suggested as a way to treat chronic pain.
Even though the tweets are full of negative sentiments, words like “relief,” “recovery” and “support” are also used. So there might be light at the end of the tunnel for some of those living with pain. As I wrote in my first blog post, there are likely more than a billion people in pain worldwide (about 1 in 5 people). Therefore, even slight progress in the development of novel treatment strategies is worth celebrating. As it has sometimes been said, “a little progress each day adds up to big results.”
Simona Denise Frederiksen, postdoctoral associate, University of Calgary, Canada.
Working With, Instead of Fighting Against, the Chronic Pain Monster
Throughout my posts during my time here as a PRF Virtual Correspondent, I have been open and direct in talking about my battles with the chronic pain monster (see “Too Young” to Be in This Much Pain, Fighting the Chronic Pain Dragon, and Living With the Chronic Pain Monster).
Well, today my pain monster is rearing its ugly head. It does not care that I have deadlines to meet and things to do. When I try ignoring it, it sinks its teeth deeper into me, demanding to be acknowledged.
I have also openly discussed my own use of prescription opioids as part of my toolkit in fighting the pain monster (see A Researcher, a Pain Patient, and an Artist and Understanding the Failure of Translation in the Pain Field). But this is such an important topic, so I feel the need to dig a little deeper into it.
In the past, I too felt shame in utilizing opioids as part of my pain management regimen. When needing to take my dose in public, I would worry about making sure no one would see me, as if I were inherently doing something wrong in taking medicine that allowed me to carry on with my day.
I refuse to feel ashamed of using a medication, especially when all others have failed and better options are currently unavailable. If it were not for my opioids helping me tame the pain monster today, I would not be able to stare at the bright computer screen long enough to write or push past the throbbing, spontaneous burning and stabbing sensations felt throughout my entire body. Before I took my medication, I was nearly unable to move my neck, and I could not close my hands all the way. I felt like the tinman from The Wizard of Oz – simply substitute the oil needed for stiffness with my pain cream.
Just thirty minutes ago I was on the verge of tears because I physically could not function at high enough levels to be productive. I had to be in complete darkness and silence, so that meant not being able to use a phone or look at my computer screen (sensory sensitivities are a common occurrence in migraine sufferers.)
All day today I have been trying to type this post. But, for the last four days, I have been dealing with fluctuating between a minor headache and a throbbing migraine accompanied by a full-body pain flare-up. I carved out time in my schedule specifically to write this post, so I grew increasingly frustrated with myself as the clock ticked by while the page remained empty.
I felt as if I was letting myself and others down. I wanted so badly to work, but my body was just not having it. I put so much pressure on myself to always be this eloquent weaver of words, to prove to the world that despite my pain I can do all the things and more that “everyone else” can do. I think a lot of chronic illness patients, whether they live with mental or physical disabilities, know this internal turmoil all too well. We do not want to seem lazy, or unaccountable, but the unpredictable nature of the chronic pain monster means we cannot always plan accordingly.
As the hours kept passing by, I began to realize the irony in my situation: My chronic pain was preventing me from writing a blog post about chronic pain. The brain fog, another common experience for chronic pain patients, made it difficult to think, so piecing together a cohesive post felt near impossible to do. Interestingly, the phenomenon of brain fog has also been noted in COVID-19 patients, both during their illness and in the months following recovery.
While jotting down my scattered thoughts and emotions accompanying my pain in real-time, I realized my post had practically written itself. In using my own lived experience as the building blocks for my writing, I decided that today, I am going to work with my chronic pain monster instead of solely fight against it.
Sarah D'Angelo, undergraduate student, Rutgers University, US.
As we all know too well by now, one of the major co-morbidities that increase the risk of a severe Covid-19 infection is obesity. Unfortunately, more than 50% of adults worldwide today are either overweight or obese. Obesity is also accompanied by a higher incidence of heart disease and type 2 diabetes, both of which are two of the leading causes of death worldwide.
The rate of obesity is increasing alarmingly, with rates of childhood obesity going from 1% in 1975 to approximately 7% in 2016. It is estimated that most overweight children will grow to become obese. These disturbing trends could be attributed to the higher consumption of junk food because of its prevalence and lower costs, and the more sedentary lifestyles that we lead today. It does not help that many of us have been working from home for over a year now and this Is likely going to last far beyond the pandemic. Therefore, the already staggering upward trends are likely to become even worse.
Why do we as pain scientists have to care about this?
It turns out that obesity and chronic pain are highly correlated. Individuals who are obese are more likely to complain of low back pain than their normal weight counterparts. A large-scale survey of over 1 million people showed a linear relationship between body mass index (BMI) and rates of recurring pain. Compared to those in the normal BMI range, individuals who are overweight reported 20% greater rates of recurring pain, while those with class 1 obesity reported 68% more recurring pain complaints, and so on.
In addition, many individuals who are obese are also either diabetic or pre-diabetic. Diabetes, pre-diabetes, and hyperlipidemia are direct risk factors for developing a peripheral neuropathy called diabetic neuropathy that leads to the degeneration of sensory neurons in the limbs first, and can affect the motor and autonomic nervous systems in more severe cases. Diabetic neuropathy affects around 50% of individuals with diabetes or pre-diabetes and presents as tingling and numbness in the hands and feet, followed by ongoing neuropathic pain that greatly reduces the patients’ quality of life and is really debilitating.
Finally, obesity is considered a state of systemic inflammation with increased pro-inflammatory cytokines and immune cell activation in the blood as well as many peripheral tissues. Studies in rodents show that obese animals are more susceptible to prolonged inflammatory pain and that the inflammation contributes to the chronification of acute inflammatory pain, which could explain the higher prevalence of chronic pain in obese individuals.
As mentioned above, with the alarmingly and steadily increasing rates of obesity, it is likely that more and more of the chronic pain patients who walk into clinics will also be suffering from obesity and its co-morbidities, and it is imperative that we get ahead of this by improving our understanding of how metabolic stress leads to chronic pain and peripheral neuropathy. I believe that we also have a responsibility to advocate for more active and healthier habits as well as policy changes that promote access to healthy affordable food in underserved neighborhoods and taxation on junk food.
Sara Hakim, PhD student, Harvard Medical School, Boston, US.
Cold Pain in the Teeth Reimagined: A Key Role for TRPC5 in Odontoblasts
Dental injuries can lead to inflammation, which compromises dental tissues. During this process, teeth can become very sensitive to cold stimulation and the ensuing cold pain can be unbearable. Unlike the cooling-sensing mechanisms in the skin, whose understanding has deeply advanced, full knowledge of cold pain detection in teeth has remained elusive. This picture has now evolved as a group of researchers has found a key sensor of cold stimuli in teeth: TRPC5.
TRPC5 (Transient Receptor Potential Canonical 5) is a nonselective cationic ion channel that is activated in expression systems by a fall in temperature in the range of 25º-37ºC (see here and here). In the new study, the group demonstrated that genetically modified mice that do not express TRPC5 (TRPC5-/-), compared to wild type animals that do express this ion channel, did not develop a preference for sucrose consumption after being subjected to an experimental model of dental pain (increased sucrose consumption is considered a pain-associated behavior in these animals). This pointed to a role for TRPC5 in dental pain.
Next, to learn more about the contribution of TRPC5 to cold pain in teeth, the authors performed electrophysiological experiments using healthy mouse teeth, by taking advantage of ex-vivo jaw-nerve preparations from wild-type and TRPC5-/- mice. They recorded electrophysiological changes (action potentials, peak frequency, temperature threshold of activation) upon temperature falls (32º to 6ºC) in the experimental preparations. Their findings demonstrated that TRPC5 appeared to be necessary for cold transduction in healthy teeth.
The next question was where this ion channel might be predominantly expressed. The authors used experimental approaches to see whether it is located in primary sensory neurons in the trigeminal ganglia. This is the structure where the cell bodies of sensory neurons that innervate the orofacial region are located. It was baffling to see that activation of these cells by cold stimulation occurred independently of TRPC5. So where is the site of expression of TRPC5?
The authors then investigated if TRPC5 was present in the cells of the teeth. They identified expression of the channel in odontoblasts, which is a type of cell whose primary function is to secrete dentine, the mineralized tissue that forms the teeth. Furthermore, similar to the findings in animals, by looking at healthy and injured human teeth, the group identified expression of TRPC5 in odontoblasts, and this expression appeared to increase in injured human teeth. These findings might indicate that TRPC5 can act as a cold sensor in human teeth.
In summary, these findings show a key role for odontoblasts in cold transduction. As an odontoblast cold sensor, TRPC5 may signal cold within the dental structure to the nerve endings that surround odontoblasts. Thus, perhaps TRPC5 could be a drug target for the management of dentine hypersensitivity and inflammatory tooth pain.
Francisco Isaac Fernandes Gomes, DDS, PhD student, University of São Paulo, Brazil.
The Link Between Fibromyalgia, Women, and Autoimmunity
Fibromyalgia and chronic fatigue syndrome (CFS) are two sister disorders that cause long-lasting muscle pain and fatigue. They affect about 2% of the population, but there is a big elephant in the room: as many as 9 out of 10 diagnosed patients are women!
Fibromyalgia’s name suggests it’s a muscular disorder, but it’s generally considered to be a central sensitization disorder. It’s a neurological disease – or at least a disease with a large neurological component – treated largely by rheumatologists. One has to look no further than three FDA drugs approved for fibromyalgia for evidence that the medical profession doesn’t have a handle on what’s going on. The fact that none of the drugs work particularly well (30% of patients get about a 30% benefit) suggests the drug manufacturers have been looking in the wrong places for the answer to fibromyalgia.
The fact that a lot more women than men have these diseases must mean something, right? It turns out that women are immunologically set up to have more issues with autoimmunity.
You can blame the kids. The Th2 immune bias that assists with fetal health also enhances the humoral (antibody) portion of the immune system that triggers much autoimmunity. Women also tend to have more of the immune cells that participate in autoimmunity while men tend to have more cells focused on keeping the immune system in check. But there is a plus side. Women, with their revved up immune responses, are generally better at fighting off infections and cancer is less prevalent in women.
And as one may easily guess, hormones play a role as well. Estrogen, the main female sex hormone, is associated with enhanced antibody responses, and a reduced ability to filter out autoantibodies (weaker tolerance). Testosterone, the main male sex hormone, on the other hand, enhances immune tolerance (autoantibody removal) and reduces antibody production. Furthermore, there is evidence that the hormone prolactin, more commonly associated with lactation in new mothers, may underlie why women are more vulnerable to developing functional pain syndromes such as fibromyalgia.
All this seems to suggest that autoimmunity may play a role in fibromyalgia. Some researchers believe that an infection or some other factor that damages the nerves starts the autoimmune process off. The damage in and around the nerve may not be large at all but if it liberates too many antigens (the structures on cells that antibodies latch onto), trouble may follow. All it needs to wreak havoc is an antibody-dominant, poorly regulated immune system. And indeed, there is a small subset of fibromyalgia patients who suffer from small fiber neuropathy, likely caused by autoimmune mechanisms. Encouragingly, intravenous immunoglobulin appears to be an effective treatment in these patients.
Mysteries abound in fibromyalgia. Sympathetic nervous system activation, herpesviruses, mitochondrial problems, blood flow to the muscles, autoimmunity, hormonal issues, and metabolic problems have all been posited to play a role. Hopefully, in the future, my PhD work will reveal some more concrete facts about this elusive disease. Until then, I hope you stay curious and keep reading the PRF blogs. It’s been a pleasure communicating with you!
Denis Duagi, PhD student, King's College London, UK.
Precision Medicine: Diagnosing and Treating Central Sensitization
If you are anything like me, then you often visit PubMed in search of one particular paper, only to find yourself two hours and 50+ tabs later with more questions than you started with and a very overheated laptop. One of my frequently visited rabbit holes lately is the subject of peripheral versus central mechanisms of pain and sensitization. Although this is a fairly saturated topic in the pain literature, it seems that there are still so many unanswered questions, especially regarding the clinical understanding and treatment of these mechanisms. A recent PubMed binge led me to wonder: How can we clinically diagnose central from peripheral sensitization?
A recent review discussed chronic pain conditions and the tremendous variability in measures of central sensitization. Most clinical measurements of central sensitization can also reflect peripheral sensitization. Furthermore, characteristics such as sex, age, ethnicity, and race have been shown to affect symptoms of central sensitization and thus can make accurately diagnosing and treating chronic pain patients even more difficult. A patient-reported outcome known as the Central Sensitization Inventory (CSI) seems to be the gold standard for distinguishing central from peripheral sensitization. In contrast to assessments such as quantitative sensory testing (QST), which uses thermal and vibration stimuli and can reflect peripheral or central sensitization, the CSI assesses symptoms that are specifically related to central sensitization. Such symptoms include sleep problems, sensitivity to odors and light, difficulty concentrating, stress as an aggravating factor, and restless legs.
More accurately diagnosing central versus peripheral sensitization will allow for a precision medicine approach to treating pain. As the review describes, “Precision medicine refers to the ability to classify patients into subgroups that differ in their susceptibility to, biology of, or prognosis of a particular disease, or in their response to a specific treatment—and thus the ability to tailor treatment to the individual patient’s characteristics.” Therefore, by using assessments such as the CSI, clinical features of central sensitization can be used to identify patients who are more likely to respond to certain pharmacological treatments. This approach would also allow patients not presenting with features of central sensitization to be considered for more unimodal, bottom-up treatments to target peripheral mechanisms. Although precision pain medicine is still a possibility in the works, pain phenotyping seems to be the first step to more accurately diagnosing and treating central and peripheral pathologies.
Morgan Sharp, PhD student, University of Louisville, US.
It is frequently believed that activism and academia are at odds with one another. One concern is that being “too involved” or “too passionate” about one’s research might endanger scientific integrity. According to these concerns, the only academic position that can be occupied is the neutralist position, in which the academic simply follows the facts wherever they lead, free of bias or their own personal views and opinions. I believe that this view is both unrealistic and limits what an academic can and should do.
First, to be but a passive observer is not at all what an academic does to begin with. As researchers we are led by curiosity and take an active role in choosing what we want to research, how we want to research it, and who we choose as participants (to name but a few examples). It is already in these simple decisions that we can recognize a form of activism, where we make choices about where we want to shine a spotlight and invest our energy, time, resources, and expertise. Very frequently these decisions are already informed by some form of injustice or a status quo that we want to improve, be it the plight of people with pain, or some other greater goal. We ought to recognize that for most scientists the goal is not only to understand, but to understand in order to change/improve a situation that needs changing.
There are certainly enough situations to choose from. Be it changing science in general (e.g., inequality of opportunity, work-life balance, transparency, replicability, accessibility of science, etc.), greater societal concerns (e.g., global warming, economic inequality), or more specific areas of research like pain (e.g., disparities in pain prevalence or treatment, the role of greater societal problems such as income inequality or racism for people with pain). Personally, working on social and cultural factors in pain, I encounter societal and cultural challenges facing people with pain on a daily basis, and a sole focus on the individual seems both short-sighted and ineffective. All too often we realize that what our findings point to is the need for larger scale, societal changes to improve a given problem. Why can we not be the ones that advocate for these changes? Who, in fact, would be better suited than us to do so?
We are uniquely suited for this, as we have the expertise to study societal problems from a scientific perspective, ideally with the skills to minimize the influence of bias and error. Activism should not affect what we find, but what we look for. It should affect how ferociously we try to spread knowledge to produce (societal) change in the world. In the end, our research is only as valuable as the change it brings about. Our findings should come into contact with politics and policies because that is where the biggest promise for change lies. Safeguarding the scientific method in the process to ensure scientific integrity and the validity of our findings is paramount but does not rule out activism, and it does not rule out actively engaging with our findings.
It is everyone’s duty to address societal injustices. So why should the people most trained in answering questions of societal relevance on a daily basis be exempt?
Kai Karos, PhD, Centre for the Psychology of Learning and Experimental Psychopathology, KU Leuven, Leuven, Belgium.
Week 5: Wednesday, April 7, 2021
Considering Sex as a Biological Variable
A Call for Labs to Enter the Race Against Single-Use Plastics
Revolutionizing the Publication of Pain Research
Time to Reframe the Fear-Avoidance Model?
Living With the Chronic Pain Monster
Nitric Oxide and Inflammatory Pain: A Role for a Gaseous Transmitter in Pain Modulation
Personalized Medicine: Prospects and Challenges
Is There Something Sensory Neurons Can’t Do? (Spoiler: They Can Promote Cancer Growth!)
Nav1.7: Not Only Important for Pain
Painful Expression: A Brief Look at Pain in Art
Health Disparities: More Than a Healthcare Issue
Considering Sex as a Biological Variable
Let’s take a quick poll: Who has received comments back from reviewers regarding your use of only males or females in your experiments? Here’s an even better one: Who has written back to those reviewers, stating that once your data is published you will then explore the effects of your experiment on the opposite sex (all while rolling your eyes and knowing that you’ll never come around to it)?
I come from a laboratory that almost exclusively uses female rodents to study clinical interventions after spinal cord injury. Although we do have a valid rationale for this (females are easier to exercise, have lower mortality/morbidity after spinal cord injury, and are what all of our preliminary experiments have used), not assessing sex differences may actually be a disservice to both the science and clinical implications of research. Furthermore, ignoring sex differences in basic science research may actually be contributing to disparities in the treatment and diagnosis of different sexes, especially women.
A Perspective was recently published in Nature Neuroscience that called for a cultural and structural change in science culture regarding sex as a biological variable. As the authors Rebecca Shansky and Anne Murphy describe, an evaluation of biomedical publications in 2017 found that neuroscience literature has used male animals over six times more often than female animals. While this may not seem like a big deal when you are just a trainee working at the benchtop, this disparity has greatly contributed to females being much more likely to be misdiagnosed than males in the clinic.
The authors explain that most “textbook” diagnostic criteria that are learned by medical students and taught worldwide are actually more representative of men’s symptoms rather than of women's symptoms. A well-known example of this is cardiac arrest; the “textbook” symptoms are more relevant to men, commonly causing delayed treatment for women and sometimes fatal consequences. Other afflictions such as stroke and attention deficit/hyperactivity disorder (ADHD) are commonly under- or misdiagnosed in females due to their symptom profiles not matching with male-derived criteria.
By identifying fundamental sex differences in behavioral, cellular, and systems neuroscience, as well as disparities that exist in treating and diagnosing females, the authors describe the necessity for rigorous science to include males and females in experiments. The profiling of diagnostic criteria for pathologies and afflictions begins in basic science research. Therefore, if basic science research is conducted with sex bias, then clinical practices and our knowledge of medicine is going to reflect this inequality.
Instead of sex differences being a comment that we begrudgingly anticipate reviewers to bring up, we must begin contributing to this global shift in science culture. Developing better clinical practices and more accurate diagnosis and treatments ultimately begins at the benchtop.
Morgan Sharp, PhD student, University of Louisville, US.
A Call for Labs to Enter the Race Against Single-Use Plastics
The use of disposable plasticware became established in lab culture with the assumption that they guarantee sterility, speed, and cleanliness – but with little consideration of the piling waste. A daily routine in the lab will usually consist of a variety of plastic-made equipment, such as tip pipettes, gloves, or Petri dishes, for mammalian cell work and tissue culture experiments. At present, most of the contaminated waste is bagged, autoclaved, and sent to landfill, or burnt at high temperatures, thereby polluting the atmosphere. In addition, any apparel that has been in contact with non-hazardous contaminants, such as glucose or even water, will have the same fate. Worryingly, a study carried out by the University of Exeter estimates that a staggering 5.5 million tons of lab plastic comes from biological, medical, or agricultural research institutions every year, contributing to about 1.8% of global plastic waste in 2014!
At first glance, several strategies can be implemented to reduce the use of single-use plastics in laboratories. Appropriate waste management systems should be implemented in labs in order to facilitate the separation and recycling of plastics. Autoclaving should be considered as a method to decontaminate cultures, glassware, and pipettes. This provides the opportunity for plasticware to be reused and washed in the lab, but is highly dependent on energy, water, and trained staff. Yet, this would only pile on the main problem – laboratory buildings at Russell Group universities in the UK are estimated to be responsible for two-thirds of the total university energy usage. Another possibility is the substitution with glassware wherever possible, which could be washed and reused without generating more disposable waste. Nevertheless, these alternatives would require costly washing systems and personnel that impede their establishment. It also raises questions around how many times glassware can be reused without affecting the results, along with implications on the potential hazardous effects, and the availability of facilities. The need to reduce the use of plastics in research environments has encouraged researchers around the globe to find innovative solutions. A future process could be to use these PET-degrading enzymes to break down PET plastics into their monomers, which have value for making new PET, or as compounds for synthesizing other more complex molecules. This would keep the carbon present in the monomers within a circular system of use.
But in the meantime, you can still reduce the amount of waste you produce by carefully designing your experiments – by using smaller size tubes to limit the overall weight of plastic that is discarded, for example. Practice good laboratory management, and don’t order unnecessary plastic products that will never be used. If you do end up with a surplus, donate them to other researchers at your institute. Sharing is caring after all.
Sometimes, plastic waste generated from lab work may be considered unavoidable, as it comes from pre-made kits that contain plasticware such as membranes, columns, or mini-centrifuge collectors, which can rarely be recycled. As such, industry manufacturers of lab supplies must also engage with this movement. Some promising steps have been taken in this direction, with schemes like RightCycle by Kimberly-Clark Professional recycling nitrile gloves; StarLab tip boxes can be reused by the company up to one hundred times if returned; and the polystyrene boxes used to ship items are taken back and reused by companies like New England Biolabs. This can provide an incentive for researchers to become more sustainable in their approach to lab work, as it demonstrates that labs that are more sustainable save more money! Lab waste reduction is nontrivial, but it is also not mission impossible. The University of Leeds and University College London pledged to break free of single use plastic by 2023 and 2024, respectively. Additional laboratories and institutions need to follow suit in order to create meaningful change on a global level. But to be successful, we all need to play our part.
We will not eliminate all plastic waste immediately, but by taking some of the steps mentioned above we can certainly reduce the amount of waste we generate.
Denis Duagi, PhD student, King's College London, UK.
Many drugs that show high promise in mouse and rat models of chronic pain conditions never make it to the clinic. As my fellow PRF Correspondent Morgan Sharp recently discussed, the poor translation of pain behaviors measured in animals to clinical manifestations of pain in patients could be the culprit. Aside from that, species differences also play a role.
This poses the question if there are better models that we can use to study molecular mechanisms of nociception in humans. The answer is a clear yes! Although in its infancy, human induced pluripotent stem cells (hiPSCs) are finally entering the pain research arena. The beauty of iPSCs is twofold. First, they can be obtained from any cell type – at least in theory – through somatic reprogramming (A Nobel Prize-worthy discovery by Shinya Yamanaka). Second, the cells retain the person-specific genetic background including all mutations that may be relevant in causing a pain phenotype.
At this point, if someone is interested in the peripheral nervous system (such as me), these iPSCs can be used to differentiate either mechanoreceptor- or nociceptor-like cells (reviewed here) and study the function of particular channels. For example, iPSC-derived sensory neurons from patients with a gain of function mutation in the sodium channel Nav1.7 (leading to the burning pain disease “inherited erythromelalgia”) showed a less negative activation threshold of the channel. In other words, in these patients’ sensory neurons a smaller depolarization produces action potential firing.
Unlike mutations in Nav1.7 and TrkA (an important receptor for bone pain), most chronic pain depends on the action of multiple genes. This is where I see the real power of human iPSCs! The case of a patient with small fiber neuropathy is an excellent example. The patient's neuropathic pain severely affected her day-to-day activities (the patient rated the pain as 7.5 out of 10). The patient was fortunate enough that Angelika Lampert and collaborators took an interest in her condition. They could not identify any single mutation that would explain her pain phenotype. Instead, they derived sensory neurons from her iPSCs and found that they showed spontaneous activity, which they could inhibit with the antiepileptic drug lacosamide. Based on this impressive result, the patient's treatment plan was changed. Her pain rating immediately dropped and six months into therapy she still reported her pain as 1.5 out of 10 – an impressive reduction compared to pre-treatment pain scores!
I hope this example has convinced you that such systems can be adapted for high-throughput drug screening tailored to each patient. Indeed, the start-up sector is booming with companies working on such technologies. Finally, with technical advances, such as the use of microfluidic chips, one day we should be able to grow the entire human pain circuitry in a dish. But until then, we shall continue to harness the utility of animal models, because, after all, with the current technologies around iPSCs we are only looking at a single branch of the blooming cherry tree.
Larissa de Clauser, PhD from University College London, now based in Italy.
Revolutionizing the Publication of Pain Research
Evidence-based medicine has brought great improvements to how care is provided to people in pain. Together, clinicians and people in pain are encouraged to integrate the best available research evidence when making decisions about care. The ability to use research evidence depends on the transparency and openness of how research is conducted and reported. Recently, the transparency and openness of pain research has come under intense scrutiny. Reports suggest that a substantial amount of published pain research is likely to be biased, distorted, and nonreproducible.
There is a need for improvements across the research landscape to ensure that people in pain can benefit from pain research. The pain research community has lots to learn from other disciplines and the wider Open Science movement to begin addressing these challenges. Scientific journals are key stakeholders in the research landscape and could help to improve pain research by implementing practices that support transparency and openness through their publication policies.
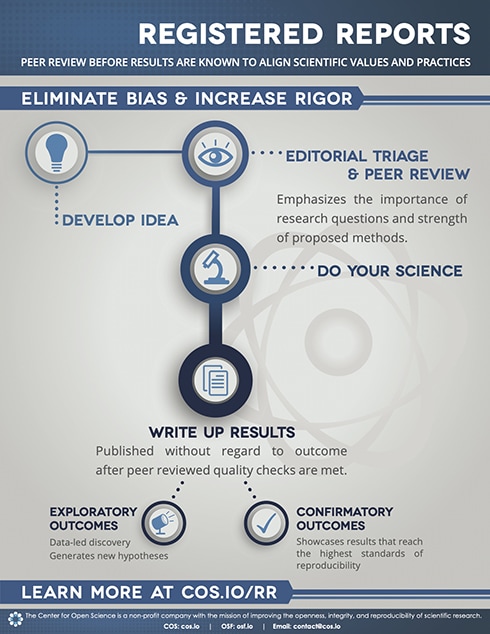
Registered reports are a revolutionary publication format that pain journals could adopt to support rigorous, transparent and open pain research. The registered reports format splits conventional peer review of research into two stages. First, editors and peer reviewers assess the value and validity of the research question and the rigor of the proposed study design and method before data is collected. High quality proposals are awarded "in-principle" acceptance, and if the second peer review after study completion verifies that the study adhered closely to the proposal and the interpretation of results is valid, the paper is accepted for publication (see infographic below).
The registered reports publication format emphasizes the importance of the research question and the quality of the research methodology irrespective of the study results. The format provides a strong incentive to plan and execute best scientific practice and protects against a variety of questionable research practices, including selective reporting, low statistical power, and publication bias.
Currently, over 290 scientific journals offer the registered reports format, but few are in medical or health journals, and none are offered by mainstay pain journals. Several initiatives have been established across medical and health research to encourage journal adoption of the registered reports format. You can find out more about the initiatives here.
The registered reports publication format is just one of many potential solutions to help improve the transparency and openness of pain research. Collaboration between pain researchers, pain journals, funders and institutions will be essential to move the pain field forward. Transparent and open pain research has the potential to benefit all that are involved in producing and using research findings and can only improve outcomes for people in pain.
Aidan G. Cashin, PhD candidate, Neuroscience Research Australia (NeuRA) and University of New South Wales.
Time to Reframe the Fear-Avoidance Model?
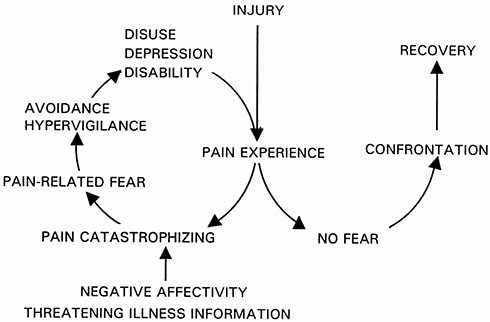
Movement avoidance is a central aspect of the fear avoidance model. This famous and very popular model in the field of pain suggests that when pain is considered as a threat, patients might develop fear of movement, which then leads to avoidance behavior and disability.
In the case of low back pain (LBP), movement avoidance can be expressed as reduced back movement and increased muscle activity. Studies show that people with LBP move slower (less angular velocity), with less movement (reduced spinal amplitude) and with higher levels of trunk muscle activity. Thus, people with LBP tend to move in a more rigid way and avoid movement of their back.
We recently published a meta-analysis in PAIN in which we assessed the association between pain-related fear and spinal movement avoidance in people with LBP. We included 41 studies and 2,832 participants.
We found that pain-related fear was significantly but weakly associated with spinal movement avoidance. The results were very consistent despite the wide range of methods of measurement of pain-related fear and spinal movement avoidance. Therefore, these results support the association between pain-related fear and an avoidance of spinal movement, as described in the fear avoidance model.
Our results also demonstrated that psychological factors and pain intensity influence spinal movement avoidance independently from each other. This result questions the sequential form of the fear avoidance model, which suggests that pain influences pain-related fear, which then influences avoidance. Therefore, our results suggest that pain does not influence avoidance through pain-related fear, and that these factors could simply be considered as cumulative and interrelated.
It may be time to reframe the fear-avoidance model. As other studies have previously suggested, a future development of the model might be to describe pain, psychological factors and avoidance behavior as factors that are interrelated, and that influence disability as cumulative factors rather than through a cyclical sequence. It would also offer the possibility to individualize this model to each patient, with a different weight given to each of these factors depending of the patient’s presentation.
Maybe the biggest challenge in doing so would be to change the universally famous graphical representation of the model.
Guillaume Christe, PhD student, Haute École de Santé Vaud (HESAV), Switzerland.
Living With the Chronic Pain Monster
Chronic pain sucks.
Saying otherwise would be to deny its very existence, minimizing the very real, very harsh reality of so many people worldwide suffering from persistent pain. As said in a previous blog (by my fellow PRF Correspondent Simona Denise Frederiksen), there are likely more than a billion people in pain worldwide (about 1 in 5 people).
Chronic pain’s relentless grasp digs its way into the lives of not just the pain patient and their loved ones, but it impacts everyone in society as well.
Pain and pain-related diseases are the number one cause of disability and disease burden globally, and the loss of productivity and costs associated with treating pain ranges from $560 to $635 billion annually in the US alone, ahead of heart disease, diabetes, and cancer combined.
In past posts, I have conceptualized chronic pain as a monster (see here and here), and I truly believe that the analogy fits.
Ask any pain patient what their priorities are when it comes to their chronic pain treatment, and besides the obvious answer of pain management, you would likely be told something along the lines of “I just want to be understood.”
But when it comes down to it, how are we to be able to understand something we cannot tangibly see and feel for ourselves? The invisible nature of any chronic illness, be it mental or physical, tends to be shrouded in mystery, thus challenging how we perceive both our own and others’ realities.
I can continue rattling off facts and statistics regarding the devastating impact of the chronic pain monster, but that has already been done. So, instead, I offer a small, yet brutally raw glimpse into the world of living with chronic pain.
This morning, I was rummaging through some old journals of mine. As I flipped through the pages, I came across the poem (see below) I wrote in 2017 when I was having one of my worst pain flare-ups to date. At the time, I had just moved across the country, so I found myself feeling isolated from those I loved most. With no health insurance, and thus no pain management, there was not much I could do but wait and hope for the pain to ease.
So, if you are lucky enough to not have had to grapple with the chronic pain monster yourself, maybe this poem I wrote will help shed light and demystify the reality of chronic pain:
Glistening beads of despair
Cascading down with remorse
Lying of their true nature
Seeming painstakingly innocent
Eluding all their essence
Breaths become painful
Lungs thicken with disdain
Eyes burn as sorrow fills them
Beats of the heart alter
Knees begin to tremble.
Reality sets in
Desperation feels interminable
Fate is oh so bitter
Emotion is overflowing
Hatred seeps from every pore.
This reflection cannot be true
This is not who I've become
This was never my intention
This will forevermore define me
This pain will always haunt me.
In the form of a distant memory
When I grow old and bitter
I will recall this time
When waterfalls slid down my face
Before I lost my sane embrace.
Sarah D'Angelo, undergraduate student, Rutgers University, US.
Nitric Oxide and Inflammatory Pain: A Role for a Gaseous Transmitter in Pain Modulation
The pharmacological control of inflammatory pain can be achieved with drugs such as opioids. In this case, their effects can be mediated by a small molecule called nitric oxide (NO). But how can NO alleviate pain? This has been extensively investigated., and here I briefly discuss how it can block inflammatory pain.
Inside nociceptors, many molecules and enzymes control the transduction and transmission of noxious stimuli. Regarding NO, there are enzymes that produce it (NO synthases) and NO-sensible enzymes (soluble guanylate cyclase), which in turn produce molecules known as “second messengers” that modify the function of ion channels in the nociceptor membrane. The question here is: Are these enzymes “druggable”? Can we target them to alleviate pain? The answer might be yes!
A drug commonly used to treat angina, sodium nitroprusside, can experimentally block inflammatory pain in rats by acting as an NO donor. It is striking to see that analgesia promoted by sodium nitroprusside is enhanced when the second messengers produced upon NO-sensible enzyme activation by NO were injected into inflamed paws in rats, whereas sodium nitroprusside analgesia was prevented when the function of NO-sensible enzymes was inhibited. These findings suggest that NO may act directly on nociceptors to reduce inflammatory pain.
Regarding the role of NO in opioid analgesia, morphine could induce the formation of the same second messengers as observed for NO. These findings led investigators to study how NO acts to mediate opioid analgesia. Similar to the case of sodium nitroprusside, the effects of morphine were reversed when the function of NO-sensible enzymes was pharmacologically blocked. Also, morphine failed to promote analgesia when NO production by NO synthases was inhibited. These observations suggest that NO is critical to the analgesic effects of morphine.
Considering the importance of NO in analgesia, one can speculate about whether designing and developing pharmacological NO modulators might succeed in pain management. It is possible that NO counteracts the enhanced excitability of sensory neurons during pain, thus promoting pain relief. So, NO modulators should be considered as possible therapeutic choices for the control of inflammatory pain.
For a historical overview of the role of NO in mediating peripheral analgesia, please read this paper we wrote on the role of the NO signaling pathway in inflammatory pain. Also take a look at two other papers here and here.
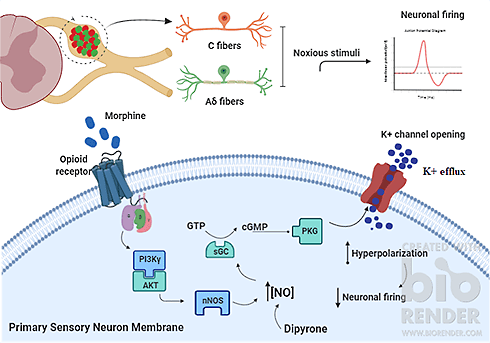
Francisco Isaac Fernandes Gomes, DDS, PhD student, University of São Paulo, Brazil.
Personalized Medicine: Prospects and Challenges
I recently watched the TED talk, “The most important lesson from 83,000 brain scans,” by Dr. Daniel Amen. It caught my attention because of my past and current research seeking to improve diagnostic success across the spectrum of disorders, from rare to common (see here and here). What particularly caught my attention was his statement that “treatment needs to be tailored to individual brains, not clusters of symptoms,” with which I agree. So, even though patients exhibit the same symptoms, the underlying cause might differ and so would the approach to treatment. Here are some reasons why I agree, along with considerations about the challenges one might face when applying individualized approaches.
Individual variability exists within biomarker studies. While searching for diagnostic biomarkers in primary headaches during my PhD studies, I noticed that the individuals included in those studies tended to show differences in their biochemical blood concentrations even though they had the same diagnosis – a migraine subtype most often. To receive a diagnosis of migraine, you need to satisfy some clinical diagnostic criteria out of a list (cluster of symptoms).
There are several ways to satisfy a diagnosis of migraine based on the diagnostic criteria, so one expects to observe differences in symptoms between patients, even for those with the same migraine subtype. This is in agreement with the content of the YouTube video, “Migraine Patient Testimonials – Wide Range of Symptoms.” Here, symptoms reported by women with migraine (who also belong to the same age category) are diverse, indicating that the cause might vary. Many of those patients would likely be assigned to the same case group in a research study. Consequently, the treatments being developed might not work for everyone, which coheres with our knowledge that drug effectiveness varies between patients (see here and here). No one is the same, not even twins. As Dr. Amen says, “treatment needs to be tailored.”
To tailor treatment strategies, the underlying cause needs to be established. Differences between patients can be explained by individual genetic makeup as well as variable environmental exposures – no news there. This means that we, as researchers, seem to know what biological aspects to look at to find the underlying cause(s) of disorders. Yet, the true cause of many disorders remains unknown. One explanation is that we are unable to separate true causal effects from noise (non-causal effects), which threatens our ability to accurately diagnose patients with both common and rare conditions.
Even though precision medicine holds promise, misdiagnosis still occurs in rare disease diagnostics where approaches often are tailored to the individual. As the condition is rare, filtering out non-causal effects can be challenging potentially because there are few other cases to compare your results with. On the other hand, for common disorders, by grouping patients together according to a broad diagnosis, we do not fully consider individual variation and, consequently, the treatments offered might be less effective. So, how do we balance the individual value gained by applying personalized diagnostic and treatment approaches and the collective value gained by developing treatments that can be used by more patients?

Simona Denise Frederiksen, postdoctoral associate, University of Calgary, Canada.
Is There Something Sensory Neurons Can’t Do? (Spoiler: They Can Promote Cancer Growth!)
I recently attended an event about cancer and the nervous system and all I could think was: Sensory neurons do it all.
Hear me out. I went into the talk thinking it would be about specific brain cancers and how they interact with the neuronal microenvironment in the brain. It's an interesting topic with many fascinating findings of its own such as cancer cell-neuron synapses and cancer cell excitability, but this is not completely unexpected; most cancers do establish relationships with the tissue they originate from.
Sensory neurons, however, are EVERYWHERE and innervate virtually all organs within our bodies, and thus have the potential of interacting with any type of cancer. Cancer cells release neurotrophic factors to attract innervation of sensory neurons, a process termed “perineural invasion.” This has been reported in many types of cancer including breast cancer, pancreatic cancer, prostate cancer, and bone cancer amongst others.
Turns out, these cancers take advantage of sensory neurons (just like they do with blood vessels) to grow and prosper (see reviews linked below for more reading on the topic). For example, the long axons of sensory neurons that travel throughout many parts of our bodies can serve as “highways” for cancer cells to travel and metastasize, an alternative to traveling through the bloodstream as a metastasis route. Another example is the direct signaling to cancer cells by neuromodulators that can promote growth and/or inhibit apoptosis. Another example is that certain neuromodulators (e.g., Substance P) promote extravasation and vascularization that can promote the neovascularization phenomenon in certain cancers. Finally, as I mentioned in my last blog, sensory neurons can directly signal to immune cells and regulate their function. Tumors can use this to their advantage as certain neuromodulators (e.g., CGRP and glutamate) can be anti-inflammatory and therefore can prevent immune cells from attacking the cancer cells.
Although this subfield of neuro-oncology remains relatively new, there are already companies that are pioneering the targeting of sensory neurons to treat different cancers. These include Cygnal Therapeutics, whose CEO, Dr. Pearl Huang, spoke at the event that inspired this blog.
It would be interesting to further study the excitability of these cancer-associated nerves and how their activity is processed in the spinal cord and higher order neurons. Do these nerves relay nociceptive signals to the CNS? Are there sensory symptoms that can be used for early detection of the associated cancers? Can some of the neuromodulators be used as biomarkers?
Relevant reviews: Nerves in CancerTumor Neurobiology and the War of Nerves in CancerRole of the Nervous System in Cancer Metastasis
Sara Hakim, PhD student, Harvard Medical School, Boston, US.
Nav1.7: Not Only Important for Pain
As pain researchers, we often find proteins that are crucial to nociception and they become known to everyone in the field. Sometimes these same proteins are implicated in entirely different processes. In the pain field, we associate voltage-gated sodium channels (such as Nav1.7) with neurons and other electrically excitable cells, and Nav1.7 is well known to us as critical for humans and animals to sense pain; when this channel is not functioning, a complete insensitivity to pain is the result.
But it's time to expand our perspective on what Nav1.7 can do. Recently, it was discovered that this channel is also responsible for the metastatic ability of cancer cells, and that it and other sodium channels like it are potential biomarkers for metastatic cancers.
The ability of cancer cells to undergo metastasis is by far the most common indicator of fatality in cancer patients (approximately 90%), so identifying the likelihood of metastasis and the mechanisms by which it occurs are of particular importance in fighting cancer.
As it turns out, as tumor cells divide and lose access to oxygen through the blood, they become hypoxic (lacking in oxygen). This triggers an electrical change in the cells where there is a loss of potassium current and a clear inward sodium current develops through Nav channels. This is due to an increase in voltage-gated sodium channels, with Nav1.7 and Nav1.5 the two most common culprits. Nav1.7 is highly expressed in prostate, non-small cell lung, stomach and endometrial cancer (it is also expressed in breast cancers); Nav1.5 is expressed in breast, colon, ovarian and melanoma cancers (see review here). The channels are expressed in their neonatal form (this is important as it is different from the mature variants), and during hypoxic conditions they are constituently (always) active. This results in a positive feedback loop where increased activity leads to increased expression and overexcitable cells. The overexcitability is what allows the cancer cells to enter the blood and spread.
The positive feedback loop is possibly a good thing for potential treatment, as blocking the activity of the channel will in turn supress its expression as well. Blocking the channel seems to have a striking effect on stopping the cancer from spreading. In animals, injecting tetrodotoxin (TTX), a strong sodium channel blocker, directly into a tumor reduced metastasis and increased the survival rate. Silencing the expression of Nav channels like Nav1.7 or blocking the channels with an anti-angina medication also reduced metastasis and tumor growth in animals.
In humans, patients treated with local anesthetics (sodium channel blockers such as lidocaine) during tumor removal surgery have a lower incidence of reoccurrence of the cancer compared to those who do not receive anesthetics. The mechanism for this is unclear as it is only a short-term administration of the drug but many cases of reoccurrence of cancer after surgery are often due to either hidden cells or a process called "showering," where the surgery causes cells to spread to other areas and grow into another tumor. It is possible that the lidocaine somehow controls that showering.
Assessing Nav channels as a potential biomarker revealed that in all cases of metastasis, Nav channels were present. In 75% of cases the sodium channel was present and there was metastasis. In 25% of cases, the channel was present but there was no metastasis. Following up with some of these patients revealed that metastasis had developed later on. This suggests that Nav channels are possible biomarkers and could be early detectors of some potentially metastatic cancers too. Some groups have actually shown that the Nav channels can set the metastasis pathway in motion.
In sum, there is evidence to suggest that the Nav1.7 sodium channel, known largely for its importance in sensing pain, and other closely related channels could also be a therapeutic target for some metastatic cancers and potential biomarkers for the metastatic potential of cancers, before they have started to spread. This is a relatively new field, so there is still much to understand, but there is a clear link between a channel that is so important in the pain field and a very different (or perhaps not so different?) process such as metastatic cancer.
Frederick Jones, CASE PhD student, University of Leeds UK and Eli Lilly & Co, US.
Painful Expression: A Brief Look at Pain in Art
With lockdown restrictions easing in the UK, I have begun to reflect on personal interests that have taken an unfortunate backseat during the pandemic. An interest that has suffered heavily due to closures is viewing art. However, with the possibility of galleries re-opening soon I thought I would begin to rejuvenate this interest and reacquaint myself with the art world, so today I wanted to take the opportunity to break away from scientific research and, for my penultimate post, talk instead about art, with a focus on the feature of pain.
From the study and exploration of pathognomics – the reading and understanding of painful facial expression – in 18th-century sculpture academies, to the photography of modern wartimes, pain and suffering have featured heavily in art in many forms and from multiple perspectives. One particularly interesting inclusion within art is found in the representation of real physical and emotional pain experienced by the artists themselves, presented both as a means of expression and as a personal therapy.
The act of creating art has long been recognized as having therapeutic benefits. The expression of emotion and physicality through artistic nonverbal means can be intensely cathartic, especially with regard to pain, which often prevents efforts to be coherent as pain is somewhat incommunicable. The expression of pain, both physical and mental, through art is therefore acting as a platform from which an individual can present one's experience. The use of art in this way is vast, so here I will highlight just a few well-known artists who have expressed pain in this way through their work.
One artist who knew the persistence of pain well and was renowned for expressing it through her art is Frida Kahlo. Kahlo suffered from chronic pain her whole life due to complications after contracting polio in her childhood. Moreover, in her youth, Kahlo was badly injured in a bus crash, leaving her with severe injuries that she never fully recovered from. She often expressed her physical pain through her art, with famous pieces such as The Broken Column conveying the feelings explicitly. In this particular piece of work, she presents herself as being held together by a series of brace supports, pierced by nails and impaled on a broken column, a clear depiction of how she felt both immediately and long after the accident.
Moreover, Andy Warhol, after being shot twice in the chest, spent a prolonged period recovering in the hospital and suffered greatly both physically and mentally for the remainder of his life. This experience and its aftermath had a major impact on his life and work, causing him to revisit themes of death that he had previously explored, moving away from larger and impersonal disasters and instead containing more private features, such as skulls as well as guns –the weapon that was used against him.
Furthermore, the 19th century French painter, Gustave Courbet, drank heavily during his life, resulting in extensive liver damage, from which he eventually died. Whilst aware of his impending fate, Courbet began to feature in his paintings the regular motif of a trout, used to represent his own physical condition. A particularly tragic example can be seen in his painting La Truite, or The Trout, which features an out-of-water fish lying bruised on the rocks and gasping for air. The fish is bleeding from its gills and pinned by a hook in its mouth, features that can be clearly understood in terms of Courbet's addiction and subsequent ailments.
Although I have only presented a few artists here today, my look into works featuring the experience of physical pain has revealed an abundance of its expression in this form. I believe, as pain researchers and clinicians, that we should all be engaging with different forms of expression, including art, if anything to allow us to better understand pain's often-uncommunicable features.
Oakley Morgan, PhD student, University College London, UK.
Health Disparities: More Than a Healthcare Issue
In my role as a medical educator for the past eight years, I have observed that there are some sessions that you know will be deep and reflective. Of these, the most common has been the session on health disparities.
I have heard some really gut-wrenching stories during these times.
One of my students had severe back pain for a few days and consulted a doctor who suggested some stretching. The pain became worse and, on repeat visits, no help was offered. So another specialist was consulted who also did not believe that one could experience such severe pain without a fall or injury.
My student developed a severe fever, started to vomit profusely, fainted in class, and was rushed to the emergency room. It turned out to be an kidney infection that had spread.
It will come as no surprise to many of you that the student was a female, in her 20s, from an ethnic minority background.
Though she has recovered from the infection, it has left severe emotional scarring, hypersensitivity to pain, and fear about future episodes. She had to drop out of her academic year, and carries a lot of anguish about not having been believed. A simple blood test would have picked up the infection and may have prevented her suffering.
Health disparity is defined as “a particular type of health difference that is closely linked with economic, social, or environmental disadvantage.”
But one thing missing from this description is that, regardless of whether you are from a developed, developing, or underdeveloped country, people from disadvantaged groups encounter health disparities for similar reasons – that is, irrespective of how advanced the education system is, how advanced healthcare policies are, or how strong economic growth is.
But better access to healthcare services will not be sufficient to reduce health disparities. Collectively, as a society, we need to take action to remove systemic and non-systemic barriers to achieve more equal health outcomes.
In an attempt to create awareness and discussion about this issue, I developed, along with my co-authors M. Gabrielle Page and Kobina Gyakye deGraft-Johnson, a factsheet on “Disparities in Back Pain,” as part of the International Association for the Study of Pain's 2021 Global Year About Back Pain.
To continue the discussion about health disparities, their impact across communities and countries, and pragmatic solutions to the problem, please join me and my colleagues for A Global View on Disparities in Back Pain, a webinar for the Global Year About Back Pain, to take place later this year on September 30. An exciting panel of clinician-scientists will be coming together to share their stories on working closely to address health disparities in socially disadvantaged groups, with a focus on sex and gender, race and ethnicity, and geographic location, including discussion of Indigenous Australian and Canadian First Nations communities.
If you would like your questions to be answered, please send them to me via Twitter @DrManasiMurthy.
Manasi M Mittinty, MD, PhD, lecturer, University of Sydney, Australia.
My son Sem is a little older than two years. He started to communicate at a very early age, trying to express himself with words. He would pick up odd words we throw around the house, both in English and in German, and build his own sentences with them. I remember the amazement washing over his face when he tried to express something in words and we were able to understand him. He would say “Auto,” and to his delight we would hand him a car. Now he speaks without end, telling us about his days, his wishes, his annoyances, and his memories. I also remember that one of the very first things he wanted to express was pain.
Obviously, the expression of pain starts early in life and takes many forms, crying probably being the most prominent one. But I distinctly remember the moment when we would help him for the first time to label pain explicitly. He had played clumsily with our cat, Juna, and she told him “enough” in the only way she could, with a light swipe of her claws. I remember the surprise on his face as he tried to slowly comprehend what had just happened and to place the unpleasant sensation that was befalling him. I believe his crying was as much from pain as it was from surprise at this sudden act. He was crying unconsolably. I was just holding him and asking whether he felt “Aua” (“Ouch”) and he cried in agreement. I told him that it would get “besser” (“better”) very soon, that the pain would fade, and that it was our cat's way of saying “no.” Language in its simplest form, maybe.
However, in the weeks to come I was amazed to witness how he integrated what he had learned. When he hurt himself anywhere he would remember and for weeks to come, and he could point at the edge of a table, a cactus, a chair, or Juna’s claws and say “Ouch.” It seemed to be the thing he remembered the best, long after we had forgotten about the odd hurt. He would use “Ouch” for other emotions as well, when he felt upset or sad. Often, after a little time passed, he would let us know that things were better. We would use the word “Ouch” to warn him of things that might hurt him like hot food or scissors. When we would experience pain he was able to pick up on it and say “Ouch.” When we told him that we were better he could seem relieved.
I study pain almost every day, and yet it could still come as a revelation how powerful it was for my son to be able to label pain, give it a place, acknowledge it, and know that it comes and fades again. How it would shape and organize his memories around the world that he was learning to navigate. It made me conscious of the weight that words have when talking about pain, it made me conscious of the ways in which we can shape another person’s experience of pain, it made me conscious of the ways that others might look at us to learn about pain and understand it.
Looking at pain through a child’s eyes is, as it turns out, a quite humbling but revelatory experience.
Kai Karos, PhD, Centre for the Psychology of Learning and Experimental Psychopathology, KU Leuven, Leuven, Belgium.
Week 4: Tuesday, March 30, 2021
Do Not Routinely Offer Imaging for Uncomplicated Low Back Pain
Fighting the Chronic Pain Dragon
The Painful Story of the Oldest Tattoos in the World
Early-Life Exposure to Noxious Stimuli: Shaping the Way We (Re)experience Pain
Self-Report, Migraine Triggers, and a Hectic PhD Defense
Both Here to Detect, Alert, and Protect: Did the Immune and Nervous System Co-evolve?
If You Want to Learn About Pain, Talk to Some Football Players
Embracing Languages for Equal Healthcare
Goodbye Genetic Determinism, Hello Epigenetics
Do Not Routinely Offer Imaging for Uncomplicated Low Back Pain
It’s not often that we see a paradigm shift in how a condition is managed. A recent practice change article in The BMJ highlights how imaging, once a routine part of the diagnostic workup for low back pain, is no longer recommended for uncomplicated low back pain. Evidence now indicates that imaging is only useful in a small (5%-10%) subgroup of people with low back for whom there is suspicion of red flag conditions (i.e., cancer, infection, inflammatory disease, fracture, and severe neurological deficits). International guidelines and “Choosing Wisely” campaigns now encourage a diagnostic triage approach to identify those people with low back pain who require imaging.
So why the change? Evidence from randomized controlled trials and observational studies indicate that imaging findings do not guide treatment decisions or improve treatment outcomes. Further, unnecessary imaging is associated with harms, most obviously increased exposure to radiation. But unnecessary imaging can also result in harms when incidental findings, features commonly seen in the population and not related to low back pain, are identified. These incidental findings can provoke worry and concern, delay low back pain recovery, and lead to further investigations, specialist referrals, and downstream interventions.
Given these advances in knowledge, you might expect that imaging rates for low back pain would be decreasing, but recent systematic reviews suggest the opposite. Imaging rates for low back pain have increased over the past 20 years, and at least one-third of all images are unnecessary.
So what’s the holdup? The article describes several barriers to change, with a lack of awareness of current low back pain guidelines and a lack of knowledge about how to apply them in practice as key contributors. The clinical environment, patient requests, and clinician beliefs may also encourage clinicians to over-order imaging. For example, a clinician may not have sufficient time to explain and justify a non-imaging approach, the patient may simply request imaging, or the clinician may believe that imaging is required to reassure the patient.
So how should practice change? The article concludes by describing four key areas where change is required to support the appropriate use of imaging for low back pain:
- Diagnostic triage and management – A thorough diagnostic triage can help clinicians identify which patients fall into the category of uncomplicated low back pain to reduce unnecessary imaging.
- Patient education – People with uncomplicated low back pain should be reassured that imaging is not required, and be provided with advice and education to support self-management.
- Communication style – Clinicians should use effective communication strategies to help people with low back pain feel reassured and supported, for example, by demonstrating empathy and using active listening strategies.
- Monitoring – Regular monitoring of image ordering practices and low back pain outcomes relative to peers can provide feedback and reassurance to clinicians on changes to image ordering practice.
There are several resources included in the article to help support practice change. Increased public awareness will be an important step towards supporting this paradigm shift in managing uncomplicated low back pain and ultimately reducing over-imaging.
Aidan G Cashin, PhD candidate, Neuroscience Research Australia (NeuRA), and University of New South Wales, Australia.
As health professionals working with people with pain, we are faced with a lot of uncertainty. Most of our diagnostic tests have poor diagnostic validity. Chronic pain conditions are still poorly understood, and we have to work with limited knowledge in our field. Many treatments have unpredictable responses, and we do not really understand why some patients improve while others do not.
Nevertheless, our culture of science and medicine pushes us to constantly seek certainty and to turn complex problems into categories and labels. We like to understand things, and uncertainty is difficult for many of us. It is often associated with vulnerability and ignorance. At the same time, patients try to make sense of their pain, and we want to respond with simple medical explanations. While it is perfectly reasonable to try to understand a problem clearly and communicate it simply to patients, is this the right way to go?
By trying to escape uncertainty, are we contributing to the over-medicalization and overdiagnosis that make our health systems unsustainable? Are we putting patients in situations where overdiagnosis leads to unnecessary or even harmful treatments?
By trying to teach healthcare students the “truth,” are we really helping them to deal with the complex problems they will face in their future practice? Are we putting them in a position where their quest for a clear diagnosis and management plan will lead to frustration and dissatisfaction when they are confronted with the real world?
By trying to give simple answers to complex problems to people with pain, are we really helping them to make sense of their pain? Are we helping them to find solutions and to manage their problem? Is the search for simple answers compatible with patient-centered care?
In their great perspective (upon which this blog post is based) published in the New England Journal of Medicine, Simpkin and Schwartzstein argue that “our need to tolerate uncertainty has never been more urgent,” and that this will require a change in the culture of our health systems and the training of health professionals.
To do that, we need to give more importance to patients’ narratives and their individual experiences of pain, and accept that there is no single truth based on objective tests, but multiple realities. We need to integrate discussion of contextual, individual, and social factors into the education of future health professionals from the beginning. We need to improve our communication and interaction skills to help patients deal with uncertainty. We need to change our language and use terms that accept uncertainty (e.g., hypothesis, plausibility) rather than ones that support one truth (e.g., one diagnosis). We need to develop critical thinking skills to question our knowledge and assumptions.
Let’s embrace uncertainty. Only then we can progress in our understanding.
Guillaume Christe, PhD student, Haute École de Santé Vaud (HESAV), Switzerland.
Fighting the Chronic Pain Dragon
Within the pain patient community, I have noticed a major recurring trend: When many of us talk about our pain, we immediately apologize afterward, as if talking about our pain is breaking some sort of unspoken social rule. We are quick to downplay our pain by adding “It could be worse,” or “Someone out there has it worse than me, so I should not even be complaining” after we describe our own lived experiences.
As people living with chronic pain, our laughter may fill the room and our smiles radiate like sunshine. What you do not see is the invisible battle that rages on every single second of every single day. We must constantly fight our pain, as it is a selfish dragon that wants to consume us, hoarding us as shiny trinkets all for itself. If pain had its way, we would slowly fade into the background, until nothing is left but an empty shell of who we once were.
It has somehow become hardwired into our minds that we should always be “strong,” always take things in stride, with grace and positivity. I am here to tell you that while that all sounds lovely on paper, it simply is not realistic. See, no one can win every single battle they face. Some days, the pain will rise as victor – and that is okay. As long as you pick yourself back up and carry on the fight, then go ahead and take the time needed to rest.
Do a quick Google search about coping with chronic pain, and I assure you that the most common advice you'll find is to “be positive.” While I wholeheartedly agree that being positive is a powerful tool for fighting the chronic pain dragon, I also believe there is a thing as preaching too much empty positivity. I understand that it comes from a good place but think back to the last time you stubbed your toe, broke a bone, or burned your hand, and then tell me how you would feel if everyone around you kept telling you to just be positive. It would be aggravating, to say the least. Some days, we just need to be able to mourn so we can accept that pain has stolen a piece from us that we can never get back.
Studies like this one (my fellow PRF Correspondent Manasi Mittinty is the first author) have found that pain patients significantly benefit both in their intensity and ability for coping with their pain if they have access to pain education. But are pain patients actually being properly educated about pain? As a pain patient who felt like I was left in the dark, I would say the answer is a resounding no, we are not.
As a pain patient, I was never really educated on any sort of ongoing pain research. I was given the bare minimum information needed for me to make “informed” decisions about which pill or injection I would choose to take for treatment. It was as if I were walking blindfolded into the abyss, just hoping something would work. Even though I have always loved science, I did not even know pain research existed until I entered academia.
I realized recently that when it comes to my chronic pain, no knight in shining armor will singlehandedly slay my dragon, majestically whisk me away, and take away all my hurt and sadness while we ride into the sunset. That is when I decided to become my own hero by choosing to dive headfirst into the world of pain research.
So how can we expect pain patients to practice the positivity we are so quick to preach when patients are not even made aware of the amazing work being done by so many scientists, advocates, and doctors worldwide? We need to do better as pain scientists and experts to knock down the walls standing between patients, scientists, and clinicians. The only way that I see a brighter future for the advancement of pain medicine, treatment, and research is for all of us to stop gatekeeping and work together as equals.
Give us the fighting chance we deserve, and in return allow us to provide you with invaluable insight that only a chronic pain warrior can have. We can all fight this pain dragon, but none of us can do it alone.
When you have exhausted all possibilities, remember this: you haven't. – Thomas Edison
Sarah D'Angelo, undergraduate student, Rutgers University, US.
The Painful Story of the Oldest Tattoos in the World
The brevity of our existence on planet Earth is what gives purpose to each individual life. It then makes sense that many of us try to leave behind a mark of our existence – by becoming a celebrity or writing a book, for instance – as we look to pass on our genes to the next generation. But how appealing would you find the idea that 5,000 years after your accidental death, one-quarter of a million people stare at your body every year?
This is the story of Ötzi the Iceman, who in the Neolithic Age lost his life on the same mountains where I ski on sunny winter days and stroll on summer nights. Today you can find him lying still in a glass coffin in the South Tyrol Museum of Archaeology. Since he was found 30 years ago, we have gained deep insight into his agonizing death through an arrowshot, the contents of his last meal, and his medical record. Ötzi was no stranger to walking strenuous mountain terrain on a regular basis, as his knee and hip joints show signs of osteoarthritis (revealed through radiography). He also suffered from degenerative disk disease of the cervical and lumbar spine, which probably caused him some degree of pain.
Back in 3500 BC, pain killers were hard to come by. Instead, the Iceman carried the medicinal fungus Piptoporus betulinus with him (its active substance, agaricine acid, is an anti-inflammatory and antibiotic). More importantly, his body was covered in tattoos. In fact, they are the oldest tattoos we know of. There has been speculation that these tattoos were not simple decorations or tribal markings, but rather served a therapeutic purpose. Why do we think so?
There are several points of evidence. First, the 61 tattoos, which can be divided into 19 groups, have no complex shapes but are rather simple lines. Second, the tattoos are not located on exposed body parts, which suggests they are not ornamental. Most of them are located either on the back or on the legs, where Ötzi showed signs of degenerative disease. Third, half of the groups of tattoos are very close to spots that correspond with today’s acupuncture points. Strikingly, one tattoo on the left ankle is located on the “master point” for the treatment of low back pain in traditional acupuncture.
Although in Western medicine the effectiveness of acupuncture has long been debated, more and more studies investigating the potential mechanisms of analgesia in animal models are emerging. However, it is still not clear if the effect of acupuncture is any different from placebo in patients. To uncover a real effect, we are in need of more randomized controlled trials, like my fellow PRF Correspondent Aidan G. Cashin recently pointed out. One thing can be said for certain: The fact that Ötzi’s tattoos predate the first reports of acupuncture in China (100 BCE) by several thousand years, and that the technique is still used today, indicates that at least some people throughout the centuries may have benefited from it.
Larissa de Clauser, PhD from University College London, now based in Italy.
In comic cartoons, the heroes sometimes pinch themselves to tell whether they are awake or dreaming. This bit of folklore has been the subject of philosophic debates, with some arguing that “pain is a mark belonging to waking experiences and never to dream experiences.”
Seeking some empirical evidence, one skeptical sleep scientist and four of his students conducted experiments on themselves in a 1993 paper. They inflated a blood pressure cuff above the knee of their colleagues during the rapid eye movement (REM) stage of sleep, when dreams typically occur. The goal was to produce ischemia of the leg muscles, which gives rise to the feeling of "pins and needles." After waking the subjects and seeking a report on any dream experience, 31% of reportable dreams included reports of pain sensations. This showed that experimental pain applied during sleeping does not always lead to awakening, and that the pain may become incorporated into the dream.
An explanation for this phenomenon has been put forward by a study that recorded laser evoked potentials (LEPs) during different stages of sleep and during wakefulness, from two different brain regions: the "lateral pain system," including the posterior insula and suprasylvian operculum, which are thought to subserve intensity coding and localization of pain inputs, and the "medial pain system," including the anterior and mid-cingulate cortex, which is linked to the orienting and arousing components of pain. LEPs are a specific type of EEG response time-locked to the application of painful laser heat stimuli, and for this study, the EEG electrodes were placed intracranially in patients suffering from epilepsy, which allowed the researchers to know precisely when the pain-related activity occurred, as well as where the brain response was located. They found that the activity in the lateral pain system during REM sleep was similar to the one during wakefulness, but the activity in the medial pain system was decreased. The authors then suggested that this dissociation is what allows the experience of pain in dreams without subjects being alerted enough to wake up.
In another study, data collected from the dreams of burn victims while in the hospital found that 30% of dreams had an element of pain. But in some of the cases, the dreamed pain did not always correspond to the injured body locations, and even some healthy controls reported pain in their dreams, indicating that we might retain a long-term memory of pain that can be transferred into dreams. Worryingly, painful dreams are more common and negatively toned in chronic pain patients, suggesting that, indeed, pain experienced in waking life might influence the occurrence of pain dreams.
So real-life pain can be transferred into dreams either as a result of a painful stimulus occurring while we sleep or as the result of a pain memory. Interestingly, some dreamers also report pain they had never experienced in real life, for example, gunshot pain. One potential explanation from this comes from the observation that people with congenital insensitivity to pain show patterns of activation in brain networks associated with pain while seeing pain in other persons. This finding suggests that pain seen in others or in media might also account for dreams with pain sensations.
And sometimes, the pain can carry over after waking up. In one Reddit thread, a user describes a dream in which their teeth are falling out in pieces: “I could feel stinging and pulling as they fell out. I could feel the heat of the dark red blood cascading from my mouth, too.” A handful of other users commented with their own experiences of feeling pain in their dreams, which lingered after they woke up.
I’ve never had the experience of dreaming that I was experiencing pain, and I can only imagine the mental anguish of such experiences and the confusion of sustaining a dream injury that persists upon waking. For those of you suffering from painful dreams or any kind of chronic nightmares, I dug up some advice from Gardner Eeden, the author of the book Lucid: Awake in the World and the Dream. He suggests employing a technique that has worked for him and others: taking control of the experience through lucid dreaming techniques. In a lucid dream, you are aware that you are dreaming and can manipulate and change your environment. “Picture yourself in the dark house but make it light. Something reaches out. Catch it, smack it, yell at it. Imagine what it might really be and give it a face. Don't tumble. Stand firm and confront it. Change it. Convince yourself it has no power over you.”
Denis Duagi, PhD student, King's College London, UK.
Early-Life Exposure to Noxious Stimuli: Shaping the Way We (Re)experience Pain
Chronic pain affects one in five adults globally. Many mechanisms account for chronic pain, ranging from peripheral nerve damage to central sensitization. Because of the heterogeneity of the causes of pain, effective therapies are still lacking. Additionally, studies of infants show that exposure to noxious stimuli at early time points in life increases pain susceptibility in adulthood (see here for a review). How does this happen and why should we care about it?
The development of sensory processing depends on a balance between noxious and innocuous sensory input from the periphery to the central nervous system after birth. Abnormal patterns of sensory inputs from tissue damage lead to long-term modifications in somatosensory processing, nociceptive transmission, and analgesic responsiveness in rodents. Tissue injuries lead to functional changes in pain pathways that last into adulthood when they happen at critical time points.
Studies report that hind paw incisions during infancy are associated with increased reflex pain sensitivity, increased thermal sensitivity, and altered descending pain modulatory systems in adult animals. Further, repeated touch and needle-prick stimulation in the neonatal period alters adult spinal sensory neuron sensitivity to innocuous and noxious mechanical stimulation. Likewise, joint inflammation during infancy has been shown to induce acute pain hypersensitivity in juvenile and adult rats.
The mechanisms that explain changes in the nociceptive system caused by noxious inputs during early life are: 1) Lower levels of endogenous opioids in areas related to nociceptive transmission and processing in the adult brain. Endogenous opioids can activate pathways that modulate spinal nociceptive circuits, such as the brainstem descending pathway. Therefore, hind paw incisions can interfere with the normal maturation of nociceptive circuits, leading to increased pain sensitivity. 2) Damage to peripheral nerves from inflammation or skin incisions during development can increase axonal sprouting and hyperinnervation of the damaged area. This is critical for the transduction and transmission of nociceptive signals because it increases the sensory input from damaged areas to the spinal cord.
In real-life contexts, newborn children admitted to intensive care units can receive up to 14 painful procedures without adequate preemptive analgesia, not to mention the everyday handling and tactile stimulation they receive, which has been associated with altered behavioral response to pain later on in life. Finally, infants who were exposed to surgical procedures during the neonatal period might require higher levels of analgesic drugs in subsequent surgeries.
Thus, the occurrence of early-life painful events can change and shape the way we experience pain in adulthood. More studies looking into the long-term effects of pain in early life could contribute to clinical approaches for the treatment of children and adults with a history of painful experiences early in life.
Francisco Isaac Fernandes Gomes, DDS, PhD student, University of São Paulo, Brazil.
Self-Report, Migraine Triggers, and a Hectic PhD Defense
When you talk to people with migraines, you will learn that various factors seem to be involved in triggering an attack. A trigger can be anything from fatigue and depression to hunger and drinking alcohol. Most times a patient’s trigger profile is established based on self-report rather than direct observation or the use of physiological measures.
Self-report has received loads of criticism and was among the topics discussed during my PhD defense. The day of my PhD defense felt like running a marathon (even though I have never done so, so I wouldn’t really know). I flew in from British Columbia not long before my defense in Copenhagen – not the best circumstance, really. I was not able to think straight due to a lack of sleep and jet lag on top of the mandatory exam anxiousness, at least for me.
I am trying to recall the discussion from that day and remember it to be something like, “How can you rely on studies that have used self-report to identify triggers when it is subjective?” Considering my state of mind that day, it could have been phrased differently, but the point stays the same. The focus was on primary headache triggers. There are several questions worth asking when diving into studies seeking to identify such triggers. For example, have the patients continuously observed a link between their attacks and certain triggers? Does the trigger evaluation only focus on the presence or absence of triggers prior to a headache attack or also on the degree of trigger exposure? And how do you know if different patients would score the same experience in the same manner on a self-report measure?
It has been argued that self-report gives you unreliable data. However, self-report is widely used in healthcare and often with great success. Especially for conditions with neurological abnormalities, which can be hard to measure, the more we listen, the more we find out. The key is that, rather than eliminate the use of certain techniques due to a potential bias, instead pay close attention to the methodological strengths and limitations of each technique and make it clear to the reader how to interpret your findings. You want to make sure to limit bias, which can distort study findings.
As I am interested in identifying valid diagnostic multi-biomarker panels for primary headaches, triggers have been on my mind a lot lately, as they are known to alter fluctuations of signaling molecules (see here, here, and here). This could potentially interfere with laboratory measures of molecular biomarkers in bodily fluids. We know very little about how triggers alter signaling molecule fluctuations in the context of primary headaches but asking and answering cell biology research questions will likely help us to learn more about underlying mechanisms and causes. As I expect triggers to negatively affect biomarker testing, I want to find ways to accurately identify triggers and measure trigger exposure in patients.

Simona Denise Frederiksen, postdoctoral associate, University of Calgary, Canada.
Both Here to Detect, Alert, and Protect: Did the Immune and Nervous System Co-evolve?
As I mentioned in my introductory blog post, I find the parallels between the immune system and nervous system particularly fascinating. They both have the capacity to detect external stimuli (e.g., pathogens, chemicals) and changes in tissue homeostasis (e.g., temperature, tissue damage).
Some questions that keep me up at night about the immune system and nervous system include the chicken and egg question: Which one came first? But also, why have two systems that serve similar roles? And did they evolve independently?
Earliest immunological and sensory processes
Let’s start from the beginning. Bacteria harbor the capacity to defend themselves against pathogens, or bacteriophages, by incorporating CRISPR sequences in their genome that they use to fight off the same viruses upon rechallenge. You could therefore say that bacteria had some form of immunity. In parallel, bacterial mechanosensation was described in 2017 and is mediated by the flagellar motor. Upon detection of a surface, the bacterium can produce motor and behavioral responses to colonize and reproduce on the surface.
Plant immune and neuronal mechanisms
A step forward from mechanosensation that distinguishes neurons is action potentials, which can be found in most multicellular organisms including fungi and plants. Interestingly, not only are certain plant cells excitable, but recent work shows that they employ glutamatergic signaling following a wound to alert the rest of the organism to danger. Additionally, plants have an immune system: They have a complement system, express pattern recognition receptors, and use RNA interference to prevent pathogens from replicating.
Appearance of multicellular immune and nervous systems
Most animals, including worms, crustaceans, and fish, actually have a brain and spinal cord structures. The first multicellular form of an adaptive immune system including antigen recognition, however, exists in jawless fish, some of the earliest vertebrates. They have the ability to detect antigens and to mount immune responses against pathogens via the function of clonally diverse lymphocytes. Invertebrates still possess elements of the innate immune system present in plants such as complement proteins and pattern recognition receptors.
Arguments for co-evolution
It seems that most organisms, from bacteria to mammals, require mechanisms to sense pathogens and insults as well as changes in their environment and execute behavioral responses. Plants need the quick action of a nervous system to alert against danger as well as the ability to recognize patterns to fight off pathogens. Jellyfish use their motor responses to fight off predators. In addition, there is mounting evidence that cytokine signaling is essential for many neuronal functions (e.g., IL-17 homolog in sensory responses in C. elegans), and neurotransmitter signaling is essential for homeostatic immune regulation (e.g., glutamatergic signaling in mast cell homeostasis in mice).
It is conceivable that both systems co-evolved and are codependent. It may even be worthwhile thinking about the neuroimmune system as a single entity where the immune system is a mobile, slower-acting branch and the nervous system is a centralized, fast-acting branch. This broader view may allow us to answer many unanswered questions in both fields and develop more sophisticated therapeutic interventions for both chronic pain and chronic inflammation.
Sara Hakim, PhD student, Harvard Medical School, Boston, US.
If You Want to Learn About Pain, Talk to Some Football Players
Sport is a huge part of many people’s lives and is often our outlet from work and daily life. Any sport has the potential to cause pain and injury, but some sports come with a much higher likelihood of pain on a regular basis. How do pain and injury, or the threat of such things, impact daily life and how people play sports? I am an American football player and coach, so here I focus on the experiences and opinion of male and female British football players; other contact sports will share some similarities and deserve their own research.
What are the considerations for playing American football when we are not being paid $15 million a year to do so? This is a high-contact team sport that consists often of one-on-one battles with an opposing player. This poses some important considerations. First, for a team sport, someone not fully engaging or just trying to not get hurt can have a large impact on the game. Second, it is hard to hide in this sport; if you are on the field, you will make heavy contact with at least one other player, and not approaching that with 100% conviction makes you even more likely to sustain an injury. Finally, contrary to popular belief, the pads and helmet do not prevent pain or injury. This is partly because most of the body is not well protected, so coming into contact with a metal helmet is painful. In addition, it turns out (and I am guilty of this), if you give someone big shoulder pads and a helmet, they will try to hit people harder and will use their head more often than without the protection. If the risk of pain and injury is such that it can significantly impact other aspects of life, such as requiring time off from work or aid from friends and family, is it worth it?
I spoke to a number of people involved in the sport about this and solicited their opinions and experiences on how the sport has impacted other aspects of their lives. Here's what they told me:
“I could never let my teammates down. I am committed to my team. If I wasn’t committed, I wouldn’t agree to play in the first place.” Anyone who plays a team sport can relate to this; we all want to have this mentality, and in an ideal world this would be the case. “I don’t ever want to quit but know I will have to at some point” is a sentiment that also rings true. There comes a time when we can’t do it anymore; this comes sooner for some people, and for some it is sudden, and for many reasons. Was the pain from one bad injury too much to handle? At what point do we get fed-up of feeling like we can’t move the morning after? Many people told me things like, “I can be limping through the week for three to four days, just from repeated blunt force trauma”; “I still can’t straighten my arm”; “some weeks, I am constantly in pain.” Even for the most dedicated players, that pain and incapacity can cause doubt to creep in.
There are often shifts in the way people approach our sport. Sometimes people stop playing after seeing a particular injury, while other times it is a life change, such as a change in their job or the birth of their child. “I have always thought the pain and injuries of the sport are worth it, though I now have a child, and I have to question if it is fair for me to risk injury that may make me a burden on my wife.” This person added that “the time will come when the risk is too great. Hopefully it won’t be for a while.” This is interesting since here is an evaluation of risk and a clear attempt and plan to re-evaluate as a person's life changes. What I learned from this and perhaps had not considered enough before, is that as humans, it's not only that our life experiences change the way we perceive pain but changes in our life circumstances can drastically change how we judge the risk of pain. I wonder how pain experienced at one time in life might be different if it were experienced at another time, or what someone’s cognitive response to pain experienced at different times may be.
When talking about a dislocated elbow, someone told me that “I had been promoted the week I did this, and my first day back in the office was two days after I dislocated it. I felt the personal pressure that I should go into work, but looking back I definitely shouldn’t have. I felt I had a point to prove to the manager that I was tough and that nothing would stop me from coming to work.” I heard this sentiment from others, too, that people often try to hide their pain from other people, particularly from work colleagues and family members, or they feel guilty that they were in pain. Some told me that they wanted to show that they would not let sport or pain get in the way of other things, while others said they felt their pain was "self-inflicted," so they couldn’t complain, or that they were inconveniencing the people around them. It is interesting that one of the main considerations was how pain may affect other people, but what about how it can affect people themselves? Many people are in the situation where “if I don’t work, I don’t get paid.” But the main effect that pain had on the people I spoke to was frustration, mostly at being unable to do simple tasks or being an inconvenience to others – a sentiment I can relate to.
The long-term impact of the sport can be quite unexpected. I heard an account of a four-inch skull fracture from over five years ago. Continuing to play and work with the injury repeatedly caused the muscle around the injured area to swell significantly, which still requires a regular Botox injection into the muscle to reduce the pressure on the brain and resulting migraines and dizziness. Indeed, everyone I spoke to expressed concern about the effects of concussion and the strange effects on memory they have begun to notice over time.
I am very grateful for the experiences and opinions I heard. It illuminates some really interesting considerations on how pain affects us in our daily lives and how that changes over time. Yet the thing I heard most consistently was that all the pain and the risk of pain is absolutely worth it! I definitely agree, though it is understandable to prioritize family and work over an unpaid hobby, especially as injuries pile on. It was interesting to hear how frustrating being in pain can be in everyday life and that the overarching concern was how the pain would affect other people.
What I take from all of this is that everyone’s relationship with pain is different, and that relationship can and will change over time. I hope everything I wrote above will be relatable for many who play sports.
Thank you to everyone who shared their thoughts with me, especially my main contributors:
Robyn Steward (Leeds Chargers, Great Britain Lions), a female American football player; John Sewell (Leeds Bobcats), a career-driven family man and general manager/lineman; Daniel Haywood (Yorkshire Rams, Leeds Bobcats), a quarterback; Chris Peel, a coach and player, and an American football journeyman.
Frederick Jones, CASE PhD student, University of Leeds UK and Eli Lilly & Co, US.
Embracing Languages for Equal Healthcare
I was once very proud of fluency in five languages (Sanskrit/Marathi/Hindi/Russian/English), but this has been shattered recently. I have noticed when speaking with family and friends that it takes me a few seconds, if not minutes, to connect meaning with words I have not used in a long time. It could simply be that since moving to Australia, I most commonly communicate in English, and sometimes weeks or even months go by before I use another language.
These experiences often create a feeling of sadness about losing connection with my roots. This is because language is more than just a tool that helps us communicate our thoughts and feelings. It is also the binding force for cultural identity and kinship.
In my work with Aboriginal and Torres Strait Islander communities in Australia (respectfully referred to as Indigenous communities), a lack of our understanding of Indigenous linguistic needs has serious consequences when it comes to pain. For example, Indigenous people may not always be forthright in reporting chronic pain. Surprisingly, this is not because they experience less pain (as widely believed) but because the scales used for measuring pain intensity, functioning (physical, emotional, cognitive), and impact are in English, with no translation to Indigenous languages.
This is a major issue because concepts of health and illness are interwoven with Indigenous communities' cultural identity, their land, family, and community. Not accommodating their linguistic needs prevents us from fully capturing the experience of pain from the Indigenous community perspective.
As Dr. Maya Angelou has famously said, “Words are things, I’m convinced…. Someday we will be able to measure the power of words.”
Language is a powerful medium for bridging communication barriers in healthcare, and embracing it is an important step towards achieving equal healthcare for all in our growing multicultural societies. We spend numerous hours speaking, reading, thinking, and writing, using language as our primary tool. If we start deliberating on the language we use to communicate our work, this will not only help us make significant advances in science but also move us towards a more inclusive world.
Manasi M Mittinty, lecturer, University of Sydney, Australia.
Goodbye Genetic Determinism, Hello Epigenetics
The idea that our genes are responsible for who we are and what we do is integral to how we perceive ourselves. However, the emphasis placed on our DNA has led to some questionable concepts, one of which is genetic determinism. Genetic determinism is the idea that our physical and behavioral qualities are dependent on and predestined by our genes.
The belief that our DNA controls all that we are and do provides a rather ugly excuse for social inequalities and allows us to remove ourselves from accountability in circumstances where moral responsibilities are required. This can often encourage sinister traits such as bigotry and sexism – of which we certainly don’t need any more.
Thankfully, scientific support for genetic determinism is now almost non-existent, and instead rapid advancements in genetics have provided novel and exciting findings that have huge implications for many fields of biological research, including pain research.
One of the most compelling advances, and a field in its own right, is epigenetics. Epigenetics can be defined as the study of heritable, but reversible, gene expression changes caused by external modification, rather than alterations to the DNA sequence itself. Essentially, our phenotype (observable traits) can be changed without adjustment to our genotype (genetic material), with the epigenome – the collection of modifications that sit along the genome – taking a dynamic and center stage position in gene expression regulation.
Furthermore, our epigenome can be altered by both the environment and life experiences, so what we eat and drink, and even periods of happiness or sadness, are capable of influencing and altering our genes. Modifications to the genome via the epigenome can manifest in many ways, sometimes minor, unnoticeable, or even positive, but at other times harmful and leading to disease.
DNA methylation is one of the most studied epigenetic mechanisms and involves the addition of a methyl group to specific sites along the DNA strand. A decrease in DNA methylation in certain regions of a gene loosens the packing of DNA, allowing for increased gene expression, while an increase in DNA methylation has the opposite effect. In the case of persistent pain, research has shown that chronic inflammation and nerve injury are able to induce changes in DNA methylation in both the peripheral and central nervous systems, which is accompanied by gene expression changes that can alter pain sensitivity.
Furthermore, epigenetic changes caused by previous life experience may be influencing susceptibility to chronic pain. Research has highlighted the impact of stress on pain, with early life adversity being shown to influence the trajectory of a persistent pain state and alter the stress axis. Within my group, the upregulation of the epigenetically regulated stress gene FKBP5 has been found to be involved in the development and maintenance of persistent pain. Additionally, a reduction in FKBP5 DNA methylation, and therefore an increase in expression, has been shown to correlate with the intensity of trauma in humans. Epigenetic modifications in FKBP5 expression due to previous stressful life experiences may therefore be a further causal factor in the development of persistent pain, acting via changes in susceptibility.
It is clear that epigenetic mechanisms are contributing to persistent pain states in some way. Mapping DNA methylation in pain-related genes during injury could be the next step towards a better understanding of its role in this context. And further developments in the field in general will hopefully rid the world of genetic determinism for good.
Oakley Morgan, PhD student, University College London, UK.
As a PhD student in a translational neuroscience program, conversations about designing “translational” experiments and “clinically modeled” protocols are common topics in my laboratory. In fact, at the Kentucky Spinal Cord Injury Research Center, we communicate on a (nearly) daily basis with clinical researchers and physicians across the street at the Frazier Rehab Center. As much as this has allowed scientists and trainees to understand the challenges that patients and clinicians face, there still seems to be a hesitation in implementing mechanistic and therapeutic findings from basic science laboratories into the clinic. This dichotomy brings up a fundamental question that we may need to ask ourselves: do we actually know what it means to conduct translational science?
The hurdles of conducting translational pain research seem to begin at the laboratory bench, as basic scientists using mice or rats have to essentially interpret the behavior they observe. As laboratory animals cannot relay their direct perception of pain or affective state, we have to avoid making qualitative assumptions about pain levels and instead use words such as “pain- related behavior” or “nociceptive behavior.” A recent position paper discussing translational pain research suggested that developing ethograms, or catalogues, of species-specific behavioral changes and comparing to clinically relevant behaviors in pain or pathological states could help scientists better translate pain in animals to people. For example, studies have previously shown that recordings of neuronal activity in the dorsal horn of rodents were correlated with pain perception reported by humans exposed to thermo-nociceptive stimuli. Furthermore, changes in pathology-related behavior in animal models may actually correspond to more chronic clinical pain conditions in patients. The relative timeline for cross-species behavioral changes and neuronal function needs to be further investigated for scientists to conduct translational experiments, especially in the pain field, which encompasses acute and chronic conditions.
Another means of integrating translational practices into research may be through back translation. As Mouraux et al. discuss, one of the major challenges in conducting translational research is the refinement of animal models. Back translation could be employed to use clinical cases to establish equivalent models of pathology or means of measuring nociceptive responses in animals, thus allowing for forward translation to progress in a more refined and clinically relevant model.
Conducting translational pain research seems to be a layered task that may be interpreted or implemented differently among individual scientists and laboratories. Both forward and back translation seem to be important to achieve translational medicine and new therapeutics for pain patients. As a translational basic science researcher myself, we must find a means of designing translational experiments and behavioral outcomes without over-speculating on the results. Starting these discussions as trainees provides a lot of hope and encouraging prospects for the future of translational pain research. For any trainees, scientists, or clinicians interested in pursuing translational research or interested in continuing this conversation, please feel free to reach out! I believe that refining translational pain research may begin with establishing a network of communication.
See you next week!
Morgan Sharp, PhD Student, University of Louisville, US.
It has been more than a year since the world changed with the dawn of what is now a global pandemic. One of the most significant changes has been to our social lives, with social distancing measures, quarantine, and curfews among some of the most efficient ways to fight the spread of the SARS-CoV-2 virus. In a time where we desperately need each other to go through a crisis, the best way to keep each other safe is to seek solitude. These sacrifices certainly place a burden on all of us, but some people are likely more affected than others – and people in pain are almost certainly among them.
In my last blog entry, I outlined the many ways in which people with chronic pain face threats to their social needs. They experience social isolation and loneliness, frequent experiences of injustice, and often depend on the help of others, such as family members or healthcare professionals. How does the current pandemic affect these social needs? (See a recent review article, upon which this blog post is based, that I wrote with colleagues.)
It is very likely that the pandemic has increased and will further increase the social cost for people with chronic pain. First, physical distancing and travel restrictions increase social isolation even further and make it harder to reach out for help. This is especially true for vulnerable members of our society, such as the elderly or people with pre-existing chronic conditions. At the same time, being forced to stay at home and combining work and family life might also lead to more stress and interpersonal conflict among family members, as well as feelings of guilt about being a burden to others.
Second, even though pain management is a fundamental human right, the mitigation of COVID-19 is currently the top priority for healthcare systems worldwide. As a result, other forms of treatment are suffering. Cancelled elective surgeries, closure of pain management services, and redeployment of clinicians to other areas of care all result in limited access to high-quality pain care. These developments, in turn, can widen inequities in relation to pain management for socially disadvantaged populations. There is a real danger that people with chronic pain are being left behind.
Third, the pandemic likely also exacerbates existing social injustices that affect pain. People who are marginalized in society (e.g., because of their gender, race, sexuality, socioeconomic status, health) are already more likely to develop chronic pain complaints; it is exactly these communities that are also hit hardest by the current pandemic. COVID-19 has disproportionately affected socially disadvantaged groups, and the ensuing global economic fallout could further magnify these inequalities in pain. The concern is that those most economically disadvantaged also run a higher risk of getting COVID-19 (e.g., because of poor sanitation or not having the possibility to effectively socially distance), are most susceptible to harm from it, and most likely to experience negative outcomes from it.
In sum, there is a great concern that the current pandemic disproportionately affects people with chronic pain. The social changes specifically might increase the prevalence, severity, and impact of chronic pain in the near future. The pandemic has highlighted the many ways in which social measures can impact health. It is now paramount to take this interaction seriously, and invest resources into both the treatment and study of populations who have been affected the most.
Kai Karos, PhD, Centre for the Psychology of Learning and Psychopathology, KU Leuven, Leuven, Belgium.
Week 3: Thursday, March 24, 2021
Why Should Pain Researchers Consider Climate Change?
Loneliness, Pain, and Inflammation
Just Drink Some (Hydrogen-Rich) Water!
Just Hanging Out: Thoughts on the Next Painful Paradigm Shift
“Too Young” to Be in This Much Pain
What Do We Know About Bone Afferents and What Do We Still Need to Learn?
Disentangling Contextual Effects From Treatments for Pain
Neurons and Immune Cells Do Talk to Each Other During Neuropathic Pain!
Can Signaling Molecule Profiles Demystify Pain and Emotion in Headache Disorders?
Can Chronic Pain Teach Us About Chronic Cough?
Cool Animals and What We Can Learn From Them
Is Caring Different From Treating?
Why Should Pain Researchers Consider Climate Change?
We work as health professionals and pain researchers because we want to help people.
Because we care about people.
Chronic pain is a huge crisis worldwide, with millions of people suffering from it. But there is another immense threat to our health and wellbeing:
Climate change.
Climate change hugely impacts the health of populations – with pollution, hurricanes, floods, heat waves, malnutrition, and population migration causing millions of death and diseases all over the world each year.
Health systems also contribute to the environmental crisis we are facing. For instance, 8% of US greenhouse gas emissions is due to the US health system. Hospitals and the pharmaceutical industry are the main contributors, accounting for 44% and 19% of greenhouse gas emissions, respectively. Therefore, the health sector has a huge responsibility to reduce its greenhouse gas emissions.
Reducing greenhouse gas emissions doesn’t mean reducing quality of care. Instead, promoting low-carbon therapies that have similar or higher health benefits than high-carbon therapies could have an important impact. For instance, physical or psychological interventions are recommended for many pain conditions and have similar or more benefit than surgical or pharmacological interventions, with a much lower impact on climate change.
Interventions for patients with pain that include potential co-benefits for health and environment could also be very valuable (see this excellent summary from The BMJ). For instance, promoting active transport (commuting by bike or foot) can have an important impact on health, with all the well-known benefits of physical activity for the prevention of cardiovascular disease, cancer, depression and pain. Simultaneously, it can have a climate benefit if combined with a reduction in transport by car.
Emerging evidence also suggests that being active outdoors, especially if exposed to a green (forests, for example) or blue (lakes, for instance) space, has additional health benefits such as improved mental health and reduced pain.
If we care for people and want to promote health and wellbeing in our societies, it is our responsibility to combat climate change as health professionals and scientists. We should prioritize the development of effective and accessible low-carbon interventions for pain conditions that will both positively impact patients and the environment.
But if we want to respond to this enormous challenge, we also have a role to play by communicating the health consequences of climate change and protesting to demand for societal and systemic changes. We need to put pressure on funders and politicians to make serious decisions for a “greener” health system. Listen to Richard Horton, editor of The Lancet, in his call for nonviolent social protest by health professionals to combat climate change.
Guillaume Christe, PhD student, Haute École de Santé Vaud (HESAV), Switzerland.
Loneliness, Pain, and Inflammation
Lockdown, social distancing rules – this past year has been pretty lonely for most of us, right? Worryingly, the coronavirus pandemic is triggering a loneliness epidemic, and as new Harvard research suggests, the rise in feelings of isolation is most conspicuous among older teens and young adults, with about 61% of American young adults reporting that they sometimes or always feel that their relationships are not meaningful and that they feel isolated.
And if you haven’t heard so far how badly loneliness can affect your health, let me do a small recap. Loneliness has been linked to everything from heart disease to Alzheimer's disease. Depression and anxiety are common among the lonely, cancers tear through their bodies more rapidly, and viruses hit them harder and more frequently. In fact, according to a meta-analysis conducted by psychology and neuroscience researchers at Brigham Young University, loneliness heightens health risks as much as smoking 15 cigarettes a day or having alcohol use disorder and is twice more harmful to physical and mental health than obesity. In simpler terms, it feels like the loneliness will kill you.
And chances are it will be a painful death. In a recent study published this month in PAIN, loneliness was found to be a predictor of future chronic pain. This association was found over a four-year period, in a sample of 4,906 older men and women, and it was augmented by high levels of inflammation. To find this link, the researchers used self-reported measures of loneliness and pain and tracked a blood test marker for inflammation called C-reactive protein (CRP), and found that a rise in CRP can predict a positive association between loneliness at baseline and chronic pain after the four-year period.
However, the biological basis of this relationship is poorly understood. Older studies found that when people felt lonesome, they had significantly higher levels of norepinephrine, one of the two main signals during the flight-or-fight response. Norepinephrine can cause a surge in a type of white blood cells called monocytes, which in turn ramp up the production of pro-inflammatory molecules such as interleukin-6 that are known to make nerves more sensitive to painful stimuli. And there also seems to be a vicious circle: inflammation can cause changes in brain activity in regions responsible for processing fear and anxiety, which could make us more apprehensive about social interaction and lead to more isolation.
To make matters worse, the rise in pro-inflammatory molecules comes at the expense of our defenses against viral diseases that stem from close social contact with other people. So, loneliness might prime you to develop chronic pain and at the same time make you more likely to catch a virus – exactly what you would like to avoid during a global pandemic!
Thankfully, we can keep social contact while physically self-isolating, thanks to social media and video communication platforms like Zoom. Indeed, it has been shown that virtual social contact can, to some extent, help to mitigate the negative impacts of social distancing for humans. So how about you get on a Zoom call with your parents or with that friend you haven’t seen in forever?
Denis Duagi, PhD student, King's College London, UK.
Just Drink Some (Hydrogen-Rich) Water!
I am a firm believer that we all have at least one individual in our life who diligently addresses any problem with the timeless phrase, “Just drink some water!” As much as I may roll my eyes at such practicality, increasing your water intake is actually a very valid suggestion, especially in the context of headaches and fatigue, and it can even improve your mood. However, while recently attempting to suppress an oncoming headache with a large glass of water, a new thought occurred to me: Could drinking water attenuate something like neuropathic pain?
While this may sound like a bit of a stretch, coincidentally a new study has been published this month that may offer some validity to my (yet again) absurd idea. Using a mouse model of chemotherapy-induced neuropathic pain (CINP), researchers found that administering hydrogen-rich drinking water significantly attenuated and prevented mechanical hyperalgesia from developing in CINP mice that received daily injections of oxaliplatin. Furthermore, CINP animals fed with hydrogen-rich water had reduced expression of inflammatory cytokines, such as TNF-α and IL-6, as well as a reduction of various oxidative stress markers in the dorsal root ganglia, compared to CINP animals fed with normal water.
One finding from this paper that particularly intrigued me was the effect of CINP on the diversity of gut microbiota in the mice. The authors found that development of mechanical hyperalgesia in response to oxaliplatin was associated with a change in composition and structure of gut microbiota, and this diversity was reduced in CINP animals that drank hydrogen-rich water. While the cellular pathways mediating these changes remain unclear, the relationship between gut microbiota and inflammatory pain appears to be strongly correlated.
In my opinion, the implications of your gut microbial diversity having the capacity to mediate neuropathic pain emphasizes the necessity for pain researchers to work in multidisciplinary teams composed of scientists outside of the typical “pain” field. While it may seem daunting to investigate pain from multiple perspectives, developing diverse teams and collaborations across multiple fields may be the best approach to treating such a widespread problem. Who would have thought what one glass of (hydrogen) water could do?!?
See you next week!
Morgan Sharp, PhD Student, University of Louisville, US.
Just Hanging Out: Thoughts on the Next Painful Paradigm Shift
The term "paradigm shift" was first introduced by physicist and philosopher of science Thomas Kuhn in his 1962 book, The Structure of Scientific Revolutions. Here Kuhn presents the idea that scientific knowledge does not progress in a linear, continuous way, but rather the prevailing frameworks themselves undergo periodic but sometimes drastic shifts. Leading perspectives become incompatible with novel phenomena and a new theory or paradigm needs to be accepted.
While describing the steps by which a paradigm shift occurs, Kuhn highlights that new ideas are often met with resistance and neglect. Think back to the familiar story of Ignaz Semmelweis, a Hungarian physician who, in 1847, discovered that washing his hands before delivering babies resulted in fewer infections in mothers and therefore fewer deaths. Semmelweis was a pioneer of aseptic technique who discovered this new and effective method through careful observation, a method that was later proven in clinical practice.
But how was this discovery received by fellow medical professionals at the time? You guessed it: with derision. So much so that poor Semmelweis was ridiculed throughout his career, culminating in a mental breakdown and eventual committal to an asylum. Thankfully, the outcome for novel ideas is less bleak in today’s scientific climate.
But what’s the relevance to pain you ask?
Well, an example of a pain perspective currently experiencing an overhaul in thought comes from the world of animal pain behavior. At present, experimenter-evoked responses are the gold standard assessments used to corroborate hypotheses, both in basic and translational pain research. But there is growing speculation as to whether these measures are really sufficient in explaining the "pain experience," drawing into question the true translational worth of the work. Furthermore, these measures raise the question as to what we are actually measuring. "Pain" is certainly a difficult response to decisively conclude from evoked responses, especially when our subjects are unable to vocalize how they feel.
Recently, however, there has been an explosion of innovation leaning away from these measures and towards a more naturalistic and therefore unbiased approach. The key to their success is in the facilitation of natural and spontaneous pain behaviors.
A recent study by Zhang et al. provides the perfect example of an innovative behavioral approach with clear perspective-shifting power. In this study, researchers identify naturalistic home-cage behaviors, performed voluntarily and established as indicators of well-being. Through unbiased, prospective observation of mice, the authors discovered an elective behavior that is reliably impacted by a persistent neuropathic pain state: cage-lid hanging. The paper presents a new kind of behavioral approach on which we can meaningfully rely and to which we should certainly pay attention.
Understandably, there is a reluctance to admit that our classic and age-old evoked measures are insufficient in capturing true pain behaviors, but is reluctance really a good enough excuse not to engage? As a predominantly rodent behavioral pain researcher myself I can understand that it feels daunting to reassess pain measures, but I must also acknowledge that the argument cannot go unnoticed, and instead of shying away, move with the shifting (and soon-prominent) paradigm.
Oakley Morgan, PhD student, University College London, UK.
“Too Young” to Be in This Much Pain
I cannot remember a time when I was not in pain.
According to my parents, when I was just two years old, I begged them to put me in dance classes after seeing ballerinas on TV. Unbeknownst to me, this was likely a catalyst that would begin my lifelong entanglement with chronic pain.
As I grew older, something felt off. Everything hurt, all the time. For many years, doctors chalked up my pain to simply being an athletic response to the intensive training that competitive dancers do.
So, I received no kind of pain treatment, therapy, or guidance educating me on how to navigate the pain I was experiencing. Constant soreness that used to ease up after a nice long bath would now persist no matter how much I followed the age-old “RICE” (rest, ice, compression, elevation) method. My occasional use of KT (kinetic kinesiology tape) morphed into a near full-body prerequisite for me to be able to perform.
For years, I pleaded with a multitude of doctors and specialists to listen to me and to look further into the all-over pain I was experiencing.
At one point, I was hospitalized due to serotonin syndrome, which is a rare, potentially fatal condition. Also known as serotonergic toxicity, it is typically induced by medication, most often anti-depressants, that cause serotonergic hyperactivity in the body. Put simply, the body becomes flooded with a toxic amount of serotonin if not caught in time. This is ironic because, similar to a high percentage of pain patients, I too have comorbid depression and chronic pain, likely due to overlapping neural mechanisms between the two conditions. Both now and in the past I have benefited from the use of a common pharmaceutical depression treatment, the SSRIs (selective serotonin reuptake inhibitors). Interestingly, the role of serotonin in the body is still largely misunderstood, but it seems to play a crucial role not just in mood, sleep, and appetite, but the inhibition of pain as well.
So how does someone so athletic and young, who usually benefits from medications that increase serotonin, end up with near-toxic levels of serotonin? Well, because my pain was not able to be “seen” in my clinical tests, it was assumed it must be “all in my head.” Instead of taking me seriously and listening when I said I had extreme levels of pain, doctors assumed it must all be just my depression’s way of manifesting itself. I was quickly put on a cocktail of various high doses of antidepressants, which ultimately flooded my body with too much serotonin.
Finally, at age 16, a sports medicine specialist took another glance at my charts. Within a matter of days, I would once again be in the spotlight, but this time it was within the confines of the small, sterile operating room instead of under the gleaming lights of a stage. Not only did I tear my left knee’s meniscus from years of hyperextension, but for years I had been walking (and dancing) on a kneecap that would shift in and out of place with each step, so there was a buildup of arthritis underneath my injuries. The arthritis under my kneecap and the meniscus were removed.
It would not be until age 18 that I was diagnosed with an autoimmune disease. The exact one changes depending on the doctor or specialist you ask, but one thing is certain: I do, in fact, clinically suffer from chronic pain.
Unfortunately, my story is not a rare one but is too often the case for so many of the millions of people worldwide who experience chronic pain – especially for us “too young to be in so much pain” chronic pain patients.
So, doctors, care providers, and family members: Please, listen to us when we say something is not right. Do not scoff at us when we say we are in pain. Just listen to us.
Sarah D'Angelo, undergraduate student, Rutgers University, US.
What Do We Know About Bone Afferents and What Do We Still Need to Learn?
As promised in my last blog post, today I am going to tell you about bone pain and in particular about bone afferents, the sensory neurons that innervate bones.
During my PhD I immediately became fascinated with bone afferents, for two reasons. First, the quality of bone pain is quite different from other types of pain (as those of you who have previously experienced a fracture will know) and I think bone afferents are responsible for this. Second, while we know a great deal about neurons innervating the skin, as they are very easy to access, we still have much to learn about afferents innervating deep tissue (in particular tissue of the mineralized type). Fortunately, Pat Mantyh and Jason Ivanusic have done tremendous work (nicely summarized here) in the last decade to improve our understanding of these cells.
From their work it emerged that, unlike neurons innervating the skin, bone afferents have a less diverse molecular profile. In fact, three-quarters of them express the receptor TrkA (tropomyosin receptor kinase A). The main ligand of this receptor is NGF, also known as nerve growth factor. As the name suggests, NGF plays an important role during the differentiation, growth, and survival of sensory neurons (if you have ever grown cultures of dorsal root ganglia neurons, you will have added NGF to your culture medium). But when NGF levels peak, for example due to tissue inflammation, this can lead to the sensitization of the sensory neurons innervating the tissue of interest and hence hyperalgesia.
It should come as no surprise, then, that this is particularly relevant for painful bone diseases (such as osteoarthritis, bone cancer, fractures and so on), as most bone afferents are sensitive to NGF. Indeed, a vast body of pre-clinical research identifies the potential of anti-NGF therapy as a treatment for painful bone disease. Unfortunately, as is often the case, animal studies have poorly translated to the clinic; only in the case of osteoarthritis of the knee and/or hip is there some evidence that anti-NGF therapy may benefit patients.
During my PhD, I wanted to gain a better understanding of bone afferents, in order to identify new targets for bone pain relief. I found that the expression of TrkA and the sodium channel Nav1.8 (which is expressed in three-quarters of all sensory neurons!) largely overlaps. Interestingly, mice in which all sensory neurons that express Nav1.8 (and hence also TrkA) are ablated can develop cancer-induced bone pain (whereas they are protected from neuropathic pain). The thought that an animal that lacks the vast majority of its sensory neurons is still able to develop any form of pain is intriguing to say the least. These findings prompt me to think that the bone afferent population, which does not express TrkA and Nav1.8, could be a key factor in driving bone cancer pain.
Now it is down to us to figure out which genes those cells express, whether the cells are myelinated or not, and if they constitute a viable target to relieve bone pain.
Larissa de Clauser, PhD from University College London, now based in Italy.
Disentangling Contextual Effects From Treatments for Pain
People with pain often want to know, “will this new treatment reduce my pain and by how much?” Last week, I touched on the need for well-conducted randomized controlled trials to provide evidence to answer questions about treatment effectiveness. By comparing the difference in outcomes between patients in two groups, randomized controlled trials can disentangle the non-specific effects associated with natural history and regression to the mean from the specific effect of a treatment on the outcome (e.g., pain intensity), and by using a placebo control, randomized controlled trials can further disentangle non-specific effects and contextual effects from the specific effect of the treatment.
Most recommended treatments for people with pain are complex, meaning they include several interacting components. Trying to design and test placebo comparisons for complex treatments is not straightforward and most researchers simply compare the complex treatment of interest against a usual care or no treatment group. In these settings, the effects associated with regression to the mean and natural history are removed but the contextual effects remain. We are therefore left wondering how much of the effect of the treatment is attributable to the specific effect of the treatment alone.
Mediation analysis is a robust method to disentangle the effects of treatments and could provide a new approach to understanding the specific effect of a treatment alone. A recent paper describes how the application of mediation analysis can isolate the specific effect of a treatment from that of contextual effects. Through a worked example, the paper describes a novel type of treatment effect, the “natural direct effect,” and how, with some assumptions, a researcher can answer the question, “what would the treatment effect be if the contextual effect was set at the natural value it would take under the control (or intervention)?” One advantage of this approach is that it forces the researcher to be clear about the contextual effect that is to be removed, for example, controlling for time with the therapist or the clinical environment.
Researchers should consider embedding mediation analyses into randomized controlled trials to better understand the specific and non-specific effects of treatments. This will require more careful consideration and measurement of the contextual effect mechanisms that are important to the treatment.
A greater understanding of the specific effect of treatments will help guide the selection and implementation of effective treatments into clinical practice, and ultimately lead to greater reductions in pain for people with painful conditions.
Aidan G Cashin, PhD candidate, Neuroscience Research Australia (NeuRA), University of New South Wales, Australia.
Neurons and Immune Cells Do Talk to Each Other During Neuropathic Pain!
Neuropathic pain results from damage to the somatosensory system. It affects up to 10% of the global population, has a strong impact on quality of life, and contributes to depression. From a therapeutic perspective, it is difficult to alleviate neuropathic pain due to its multiple underlying mechanisms. Immune cells can be a major driving force for neuropathic pain. Yet, if neuropathic pain primarily affects the nerves, why are immune cells so important?
Immune cells orchestrate responses to the molecular products of nerve damage. These cells respond to such signals by secreting cytokines and chemokines, which can evoke pro or anti-inflammatory outcomes. This happens through the recruitment of cells such as neutrophils, monocytes, and macrophages from the bloodstream to damaged regions or by stimulating the proliferation of macrophages already present within the affected tissue. Once these immune cells gather at the axonal projections or soma of sensory neurons, they can drive aberrant sensory processing, further contributing to the development of neuropathic pain. Conversely, other immune cell subsets such as regulatory T cells might have an opposite role by promoting anti-inflammatory outcomes.
As not all subsets of the vast repertoire of immune cells have a clear role in neuropathic pain, here, I will focus on two cell types with recognized roles in neuropathic pain: regulatory T (Treg) cells and macrophages. Treg cells express the cluster of differentiation 4(CD4) and Foxp3, a transcription factor, as markers. These cells can suppress the responses of the adaptive immune system by secreting anti-inflammatory molecules, including IL-10 and TGF-β.
A study last year characterized the role of Treg cells in neuropathic pain development using a partial sciatic nerve ligation model. The authors revealed that Treg cells infiltrate the site of nerve injury, counteracting pro-inflammatory outcomes. Furthermore, they identified the release of IL-10, an anti-inflammatory cytokine, as the main mechanism by which Treg cells regulate the development of neuropathic pain. Thus, this study opens avenues for immunotherapies targeting Treg cells as putative approaches to tackle neuropathic pain after a peripheral nerve injury.
As for macrophages, a recent comprehensive review from my colleagues discusses the role of macrophages present in the dorsal root ganglia. Being in such intimate contact with sensory neurons, these immune cells emerge as novel modulators of neuropathic pain. The review pointed out that these cells are involved in the pathophysiology of neuropathic pain through the production of pro-inflammatory and pronociceptive mediators; they can be activated in the sensory ganglia after peripheral nerve injury mainly by pro-inflammatory cytokines and chemokines, and could ease neuropathic pain by releasing IL-10, an anti-inflammatory cytokine.
So, the interactions between macrophages and sensory neurons during neuropathic pain could be of clinical relevance as targets for drug therapy to alleviate neuropathic pain.
To sum up, the harmony between the immune and nervous systems is disrupted during neuropathic pain. Rewiring the neuroimmune axis requires a deep understanding of the molecular signals immune cells and neurons use to communicate with each other. By doing so, we can one day target that communication to alleviate neuropathic pain.
Francisco Isaac Fernandes Gomes, DDS, PhD student, University of São Paulo, Brazil.
Can Signaling Molecule Profiles Demystify Pain and Emotion in Headache Disorders?
The easy answer is “We do not exactly know.” But instead of choosing the easy answer, I want to bring you back to the time where I was enrolled as a PhD student at the University of Copenhagen – a mixture of historic and modern buildings spread out through the city and my home for 10 years. The year when my PhD journey began was 2016. The years to follow were filled with mixed emotions ranging from excitement and enthusiasm to anxiousness and hopelessness. This is probably not very different from how other students experience their journey through the system.
Sometime during my PhD studies, I started to focus more and more on how to identify valid biomarkers to accurately diagnose primary headache disorders since this knowledge would help improve individualized treatment strategies. I wanted to help people get their life back. I was keen to find out if I could prove my hypothesis that “specific signaling molecule profiles, developed based on blood-derived biomarkers with a clinical diagnostic potential, exist in both migraine and cluster headache patients.” I did find some answers, which led to three publications (see here, here, and here).
It is always good practice to start out with a literature search, and potentially a meta-analysis, before initiating any new studies to get an idea of what is out there. That is what I did. During my search for current knowledge on signaling molecule profiles in the context of primary headaches, two molecules stood out: calcitonin gene-related peptide (CGRP; a pain signaling molecule) and serotonin (a "happiness" molecule). This was not a surprising observation since some of the most effective primary headache drugs on the market target the CGRP or serotonin pathway.
If you inject CGRP into the bloodstream, many migraine patients will develop an attack. It was therefore no shock to me when we found in our review that blood CGRP levels were consistently elevated during migraine attacks across studies. There was a lot of inconsistency across studies with regard to serotonin, yet our findings indicated that females with episodic migraine might have lower blood levels of serotonin. Based on that finding, and because serotonin is involved in regulation of emotionsas well as major depression, one wonders if this relates to the depression that some of these patients experience.
A few molecules will hardly explain the pathophysiology underlying primary headaches (or other conditions for that matter) nor do I believe that a few molecules can serve as valid biomarkers for the purpose of differential diagnosis. But I do believe that molecular profiles, rather than single molecules, potentially can serve that purpose. Fluctuations of signaling molecules have been widely described in between and during primary headache attacks (see here and here) as well as in affective disorders (see here and here). Based on my PhD studies and other literature, I believe that signaling molecule profiles can inform us about pain and emotion but exactly how remains to be elucidated.
Simona Denise Frederiksen, postdoctoral associate, University of Calgary, Canada.
Can Chronic Pain Teach Us About Chronic Cough?
If you suffer from chronic cough, you likely had a really tough time being in public in the past year. Although cough is generally useful for ridding our airways of potentially harmful or irritating chemicals or allergens, and for alerting us to an infection (such as a Covid-19 infection), cough can become maladaptive and evolve into a chronic condition where it is not triggered by a specific tussive stimulus and it greatly reduces quality of life.
Chronic cough affects somewhere between 9%-33% of people in the U.S and Europe and is often accompanied by anxiety, depression, and sleep disturbances, much like chronic pain. The two diseases, seemingly very different, share many similarities in clinical presentation and biological mechanisms, and advances in pain research may prove to be applicable to the understudied mechanisms of chronic cough.
Clinical presentation
One of the features of chronic pain patients is that they exhibit more intense pain in response to a certain painful stimulus than their healthy counterparts. This phenomenon is called hyperalgesia. Likewise, chronic cough patients may exhibit cough responses to a tussive stimulus at concentrations lower than their healthy counterparts; this is termed hypertussivity. Another feature of chronic pain is a painful sensation in response to otherwise non-painful stimuli termed allodynia. In parallel, those who suffer from chronic cough also exhibit a tussive response to non-tussive chemicals such as a perfume; this phenomenon is called allotussivity.
Neuronal mechanism
The sensory neurons responsible for relaying nociceptive signals are generally the small-diameter unmyelinated C-fiber nociceptors and the thinly myelinated Aδ fibers whose cell bodies reside in the dorsal root ganglia. In parallel, the sensory afferents responsible for cough are also of the C-fiber and Aδ fiber subsets of sensory neurons whose cell bodies reside in the jugular and nodose ganglia of the vagus nerve. Both cough and pain neurons express the sodium channels Nav1.7, Nav1.8, and Nav1.9 as well as the large-pore ion channels TRPV1 and TRPA1. Patients with hereditary null Nav1.7 mutations exhibit insensitivity to pain that can be fatal since they are more prone to burns and fractures. It is unclear but would be interesting to investigate whether these patients also exhibit a lack of cough reflex to tussive stimuli.
Common therapeutic targets?
As the race to identify novel chronic pain therapies continues, it is possible that we will also make breakthroughs in chronic cough treatments given the similar molecular pathways involved in both conditions. In fact, many clinical studies have found that medications currently used to treat chronic pain, such as gabapentin and amitriptyline, are also effective for chronic cough. Targeting some of the ion channels mentioned above remains a hot area of research in both academic and industry settings and could prove to be a useful strategy to treat chronic cough as well as chronic pain.
Sara Hakim, PhD student, Harvard Medical School, Boston, US.
Cool Animals and What We Can Learn From Them
As humans, we rely on having a combination of good senses (vision, hearing, touch/vibration, smell, pain) yet there are other animals that have evolved to use a few senses very well, sometimes to the detriment of other senses – for instance, dogs and rats have exceptional hearing and smell but poor eyesight. Some snakes even have the ability to detect infrared to generate thermal images.
Animals that have evolved to rely heavily on a specific sense become highly specialized. Researching how they become so specialized can provide insight into human senses (and it's just a fascinating topic in general). Below are a few interesting examples.
Ducks bills are like specialized fingers
Ducks forage by putting their faces into murky water and rummaging around with their bills. This can seem like they are either looking or smelling for food. On the contrary, they are actually "tactile foragers," so they are in fact using their bills to feel for food when they dunk their head in the water. In order to be so proficient in sensing touch and vibration, the number of large-diameter neurons responsible for sensing touch are increased in the trigeminal ganglion of the duck. In contrast, there are fewer temperature-sensitive neurons in the ganglia, so ducks' bills are quite insensitive to temperature.
The trigeminal ganglion is a collection of neurons for the sensing of touch, vibration, temperature and pain in the face, in humans as well as in ducks and other animals. It's interesting that ducks use their bill like a specialized finger but knowing this can also provide information about how we as humans also sense touch and temperature. It turns out that these ducks have a large increase in Piezo2 in their neurons, a protein that is important for the sensing of touch in other animals, including people.

Pit vipers create thermal images using infrared
I am particularly fascinated by this research, partly because it is a completely unique sense and yet its basis is in a protein that we as humans share (and also because there is no way I would ever get close enough to a rattlesnake to have made this discovery). Infrared radiation is in fact long-wavelength light energy; as humans we feel it as heat but cannot see it as light. But Pit vipers can "see" infrared images: they detect infrared as a change in temperature, which is combined with vision to form a thermal image.
Researchers discovered that Pit vipers have a very high level of TRPA1 compared to other snakes. TRPA1 is one of the critical proteins in humans and other animals for sensing chemicals and possibly temperature (this is still being debated). Infrared emitted by prey increases the temperature inside the Pit organ, which consists of two holes on the face with a membrane that detects changes in temperature via TRPA1; combining this with visual information makes for one of the most precise trackers of prey in the animal kingdom. The convergent evolution of TRPA1 in vipers, pythons and boa snakes means that their TRPA1 is not the same as the mammalian variety, so there is no suggestion that human TRPA1 can detect infrared. But the pit viper is still cool to consider.
Mole rats had enough of being stung by ants
Recently, a number of African mole rat species related to naked mole rats were found to not respond to capsaicin (the component of chilli peppers that makes them hot), AITC (allyl isothiocyanate) or acid (hydrogen chloride).
Upon closer inspection, the mole rats expressed a protein called NALCN at far greater levels in their pain-sensing neurons than humans and other animals. The result is that the pain-sensing neurons in these animals are perpetually depolarized and therefore nonfunctional.
It turns out that some of these mole rats share their burrows with a species of ant (Natal droptail ant) that is hyper-aggressive and has a venomous bite; these mole rats were unresponsive to this venom as well. This suggests that these particular mole rats have evolved to not be bothered by ant bites, allowing them to live in an area that lots of other animals would avoid. I suppose becoming insensitive to your neighbors is one way to solve the problem.
I hope the above examples highlight some of the really interesting mechanisms animals have evolved to help them adapt to their environments. While humans do not have these same capabilities, all of the major proteins responsible for the examples discussed above are expressed in the human sensory system as well.
Frederick Jones, CASE PhD student, University of Leeds, UK, and Eli Lilly & Co, US.
Everyone has needs. Enough food and running water, the warmth of a house, and space to rest might come to mind first. The feeling of security and safety, the freedom to do as one pleases, might come to mind as well. Very quickly, though, we might also think of other kinds of needs: The need for close relationships with others, in families, friendships, or romantic relationships. The ability to meaningfully engage with others. Kindness, love, and respect. Being seen. Being believed. To be treated fairly by others. A certain degree of independence and autonomy from others, while at the same time being part of an enriching social network of people. As humans we have evolved to care deeply about our connection with each other. Some of our most fundamental human needs are social needs.
Pain has always been recognized as a threat to one need above all else: Safety. Pain has the amazing ability to alert us to threat, to make us pay attention, and to motivate us to seek safety and rest. Pain is incredibly useful in this way. However, safety is not the only human need that is affected by pain. Our social needs are affected as well (see a topical review on this topic that I co-authored and that inspired this post).
Our need for self-sufficiency and autonomy can be challenged by pain, especially when it is chronic. Suddenly we must rely on others, be it family, doctors, or strangers, for help and explanation. Suddenly we are out of control. Suddenly we feel like a burden or a hindrance, slowing things down rather than moving them along. In extreme cases, when pain is inflicted by others, when we find ourselves becoming a victim, feelings of control can vanish completely. A feeling of helplessness, of being at the mercy of others, of having to rely on others, of being out of control, are common experiences when pain is present, especially when the pain lasts.
Pain can also place us on the outside. It makes it harder to engage with others and forces us to stay at home rather than go to work or meet with others outside the house. It can be lonely, and especially so when others start to see you differently. Sometimes pain is not believed, or even worse, there are accusations of malingering and exaggeration. Sometimes pain is not seen, not recognized, and not respected. Especially when the proper “proof” like a broken arm or an outside wound are missing. Or when pain does not go away.
Lastly, pain is seldom fair. We like to believe that people get what they deserve but that is not the case. Pain can be the consequence of chance or of other people’s negligence as in the case of an accident. Sometimes being vulnerable about our pain is punished by others, or we are treated unfairly because of it. We might blame ourselves for our pain or we might blame others for it. In any case, long-lasting pain never feels just. Not one person deserves it.
Pain threatens more than just our need for safety. It can threaten our social selves, the very way we connect to others in the world and how they connect to us. There is a social cost to being in pain. And this cost matters because as we begin to realize more and more, when our most fundamental needs are threatened, it threatens our health as well.
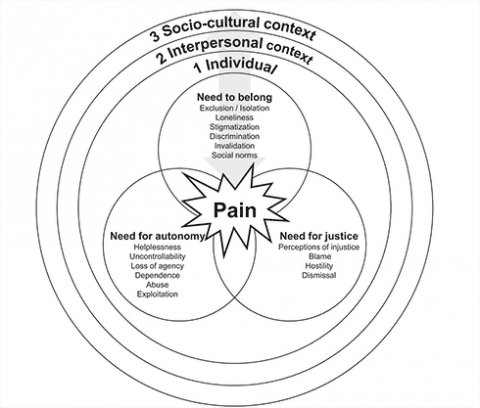
Kai Karos, PhD, Centre for the Psychology of Learning and Psychopathology, KU Leuven, Leuven, Belgium.
Is Caring Different From Treating?
Have you ever wondered what caring for patients truly means? Is it different from treating them? I have often pondered this dilemma. Fortunately, on multiple occasions I have had the chance to see what caring looks like from patient's perspective.
During my PhD I was fortunate to collaborate with Dr. Natasha Curran and Prof. Amanda C De C Williams on a project conducted at the Pain Management Centre at University College Hospitals, London, UK. This study aimed to elicit an as honest as possible account of chronic pain patients’ experience of receiving care at the center.
After carefully contemplating the pros and cons of several methods, the decision was made to use the novel approach of asking patients to write a “Letter to a friend” either recommending or not recommending the center.
In total we identified six different themes from the letters: staff attitude and behavior; interactions with the physician; the importance of a dedicated pain management center; personalized care; benefits beyond pain control; and recommending the pain management center.
But what has stayed with me from those letters is the recurring reference to the positive experience patients had on their visit to the center. The letters described at length how kind and helpful both the staff and doctors were, how the staff often went out of their way to make patients feel comfortable – even offering them a cup of tea and biscuits at the end of their session. The letters that were pro-clinic described the center as “an oasis” where patients received ongoing support even after completing their treatment.
On a different occasion, I was attending clinic rounds and was scheduled to see an elderly female patient diagnosed with severe vitamin B12 deficiency. Her case was flagged because despite being on treatment for B12 for nearly a year there was no change in her symptoms.
During the consultation the patient told me about her visit to India and how much she and her husband had enjoyed it. I picked up from our conversations that she had lost her husband and was struggling to cope without his companionship. Although there were no improvements in her presentation, she said she looked forward to coming to her appointments.
I requested that she bring the tablets to our next consultation. The moment I said that she took the tablets out of her handbag and put them in my hands. To my surprise the label said B6! She had purchased these tablets since they were on discount and had been taking two tablets instead of one to make up for B12.
The solution was really simple: I ordered some vitamin B12 tablets from the clinic to be delivered to the patient at her home. The staff booked additional phone and in-person appointments for regular follow-ups and we also involved her in a physical therapy class held at the clinic every Thursday night.
The patient showed tremendous improvement in the year that followed. As much as my training tells me that the tablets helped, I truly do believe that this success also came about because of the amazing staff being more attentive to her needs.
This takes us back to the issue of “caring” versus “treating” and my experience makes me believe that caring is in fact a step ahead of treating. But if you ask me what differentiates these two constructs? I am not sure. But I think a good starting point could be the focus on the person instead of on the symptom.
What do caring and treating mean to you? Let me know on Twitter @DrManasiMurthy
Manasi M Mittinty, lecturer, University of Sydney, Australia.
Week 2: Tuesday, March 16, 2021
A Lesson in Pain Assessment From Count Rugen
An “Objective” Assessment of Pain?
The Discreet Power of Music: Insight Into Sound Therapy as a Pain Treatment
The BonePain Patient Ambassador Group
Are You With Joe Fox or Sheldon Cooper?
“Outcome Measures Measure Outcomes – Right?”
Orofacial Pain – From the Bench to the Dental Chair: An Integrated Experience
Understanding the Failure of Translation in the Pain Field
Is My Pain Gone or Am I Just Hungry?
A Lesson in Pain Assessment From Count Rugen
“I’m sure you’ve discovered my deep and abiding interest in pain. At present, I’m writing the definitive work on the subject. So I want you to be totally honest with me on how the machine makes you feel…. What did this do to you? Tell me. And remember, this is for posterity so be honest – how do you feel?” – Count Rugen, The Princess Bride.
This weekend I decided to take an evening off from grant writing and staring at a laptop screen, and opted to stare at a larger screen instead. While scrolling through movies I had already watched and re-watched during quarantine, I came across a winning classic: The Princess Bride. I sat back, expecting the movie from my childhood to be a relaxing distraction from the growing to-do list in my lab notebook, only to find myself (once again) thinking about science and somehow resonating with the evil pain researcher and villain, Count Rugen. While I am by no means in support of building a life-sucking machine, Count Rugen’s diligence in accurately assessing and quantifying pain was certainly relatable and a mutual endeavor of my own. Therefore, I couldn’t help but watch this iconic scene and contemplate: How can we better assess pain?
Accurately quantifying pain in those suffering from acute or chronic conditions has remained a difficult task for both patients and researchers alike. Not only is pain an individualistic experience, but it is also subjective and dynamic, thereby making it difficult for patients to describe or physicians to assess using a numerical scale. Combine all of this with the fact that pain is not outwardly visible to the human eye, and we have quite a challenging task amongst ourselves.
I will admit that pain assessment has progressed beyond the classic 0-10 smiley face pain scale (formally known as the Wong-Baker FACES Pain Rating Scale, by the way). The McGill Pain Questionnaire (MPQ) is a commonly used, self-report measure of pain that assesses both the quality and intensity of an individual’s pain by addressing three primary components: What the pain feels like, how the pain changes with time, and the strength of the pain. The MPQ is useful for monitoring pain over time and can determine the effectiveness of an intervention at attenuating pain by yielding numerical indices. However, descriptor-based questionnaires such as the MPQ can present a problem in terms of readability, as patients may have difficulty in understanding the verbal descriptors or feel that descriptors do not fully encompass the entirety of their pain.
In an effort to address the dynamic and anatomical reporting of pain, smartphone apps are now being developed to include a pain mannequin as a novel approach to measure pain on a daily basis. By shading or marking painful areas on a human body, this tool can capture multiple dimensions of subjective pain, including localization, intensity, anatomical distribution, and segmental patterns. Furthermore, there has been a movement to utilize art, painting, and pictorial representations as a means for patients to describe their pain. While paintings or drawings may not always provide anatomical descriptors of pain, art provides an affective description of pain that can offer insight into the patient’s subjective experience while providing therapeutic relief.
Although these means of pain assessment have contributed greatly to our clinical understanding and treatment of pain, I believe that we must continue to generate discussions regarding how we can better assess pain on an individual basis. As Count Rugen states, “… pain is the most underrated emotion.” To accurately assess and treat pain we must create a means of evaluation that is as dynamic as the pain itself. PRF Readers, I hope you join me in aspiring to create novel assessments of pain, and I encourage you to take occasional breaks from science to watch classic movies from your childhood. Although I don’t expect you to frequently resonate with the evil villain, you may be surprised to find yourself jotting new ideas in your lab notebook before the movie ends.
Until next time,
Morgan Sharp, PhD student, University of Louisville, US.
An “Objective” Assessment of Pain?
There is an ongoing quest to find an “objective” measure of human pain. Often what is meant by this is to use some form of biological measurement, such as brain imaging data or psychophysiology (e.g., skin conductance data), to assess pain rather than relying on self-report, which is most commonly used. The underlying assumption here is that reporting pain is “subjective” and hence, less reliable, or accurate, than more “objective” alternatives. There are several problems with this idea, and these problems are crucial.
First, novel, objective measures of pain frequently aim to “filter out noise and error” that allegedly plague self-report. Such factors could be expectations about pain, fear of pain, mood, or catastrophizing about pain. In short, one wants to correct for the subjective nature of pain itself. However, we spend decades showing that, in fact, all of these features influence a person’s pain experience. They are a feature of pain and not a bug. The experience of pain is subjective by definition; it is a complex interaction among biological, psychological, and social factors. A valid measurement of pain must account for these factors, not control for them. A perfect brain assessment of pain, for instance, would have to entail the several types of subjective influences known to affect pain.
Second, most new “objective” measures of pain are validated by using self-reported pain itself. As such, any new method’s validity is tied to the validity of self-reported pain (which is how it should be). An alternative approach I have encountered is to validate new assessments of pain purely based on the intensity of a painful event (e.g., the intensity of an electric shock) and circumvent self-report entirely. This is even more problematic, as it marks a return to days past where a biomedical understanding of pain proposed that pain is nothing more or less than damage to the body. This idea is harmful and long outdated.
Why does this matter? The great danger here is that frequently, novel, “objective” measures of pain are proposed as a replacement or improvement of self-report. That means, when we then find the inevitable divergence between self-report and these new measures, the inescapable conclusion is that self-report, a person’s own account of their inner experience, is less trustworthy and reliable than the alternative. People with pain are already frequently disbelieved and invalidated or, even worse, accused of feigning, especially when there are no outward signs of bodily damage, as is often the case with chronic pain complaints. Certainly this is not a trend we want to accelerate.
There is a place for alternative pain assessment methods. Sometimes we cannot rely on a person’s self-report, as in the case of a baby, or a person suffering from dementia, in which cases we must rely on an alternative. However, above all, we want to strive for an assessment of pain where a person’s personal experience always takes center stage, and a person’s own account naturally seems like the very best way to do so.
Maybe it is high time we consider that we might have already found the best, most affordable, and most accurate way to assess someone’s pain experience: Simply asking someone about their experience, listening, and trusting their answer.
Kai Karos, PhD, Centre for the Psychology of Learning and Psychopathology, KU Leuven, Leuven, Belgium.
The Discreet Power of Music: Insight Into Sound Therapy as a Pain Treatment
One of my favorite-ever pieces of music is the 1975 masterpiece, Discreet Music, by musical innovator and father of modern ambient composition, Brian Eno. I often listen to this record when I am feeling overwhelmed, utilizing the sways of its slow repetitive sound as a kind of meditative tool, serving to distract me from thought and rid me of anxieties.
Regardless of what type of music you listen to, you have likely also experienced a physiological response evoked by sound, perhaps using music as a device to relax, to focus while working, or for a rush of motivation while you exercise.
The ability of music to have such physical effects and thus serve as a therapeutic form has always fascinated me. My scientific nature makes me question the function of sound as a comforter, asking what physical attributes underlie this ability and the biological mechanisms at play. Is it the tone, pitch or frequency of a sound that lends itself to these effects? Are sound and music stimulating serotonergic and dopaminergic pathways? Can sound activate certain brain regions and serve as an inhibitor of maladaptation in illness and disease?
In addition to thinking about the physical and biological attributes of sound, I often wonder whether chronic pain sufferers would benefit from sound therapy. The pharmaceutical treatments for chronic pain conditions frequently fall short of effective pain management, so could sound be a successful therapeutic accompaniment or even an alternative? Though research into sound therapy for pain is lacking, perhaps due to the holistic connotations, I thought I would share some interesting findings with you.
The key to sound therapy lies in accessing naturally occurring brainwave states using low-frequency sounds. There are four low-frequency brainwaves, which correspond to the electrical activity emanating across the brain: beta, alpha, theta, and delta. Each low-frequency brainwave corresponds to a different and decreasing range of frequencies, respectively, and are active at various states of alertness.
The alpha range is well studied, with waves in this frequency being shown to stimulate the serotonergic system and reduce production of the stress hormone, cortisol. Alpha brainwaves have also been found to relate to an individual’s experience of pain, with higher-end alpha frequency activation related to an increased resilience to a painful stimulus and lower-end frequencies to a higher susceptibility to pain. These attributes place the alpha brainwave as an active participant in the suppression of pain. Furthermore, low-frequency brainwaves are also known to stimulate the release of nitric oxide which, although playing a complex role in the modulation of pain, has been demonstrated to inhibit peripheral and central nociception and mediate the effects of analgesics.
Low-frequency sound stimulation itself has also been examined in the context of pain conditions, with a 2015 study uncovering therapeutic benefits of sound therapy for fibromyalgia. Patients in this study showed significant improvement in symptoms following a twice-weekly treatment with low-frequency sound. Although the biological mechanisms underlying this effect were not determined, the benefits for such a debilitating condition are promising, and the research alludes to the possible therapeutic potential of sound for chronic pain conditions. Further research in the field may help to uncover the biological mechanisms that underlie this effect.
As a final thought, I would like to reflect back on Discreet Music. It is interesting to note that the inspiration for this record came while Eno himself was hospitalized after a car accident. Although written as an experimental piece, you can’t help but wonder about the unknowing but intuitive therapeutic underpinnings of its composition, and whether Eno has inadvertently written a piece that accesses those low-frequency brainwaves.
Either way, it is a joy to experience the comfort of such music, so let us open our ears and keep listening.
Oakley Morgan, PhD student, University College London, UK.
 Everybody has heard this advice: “Be careful when you lift,” “try to keep your back straight.” These messages have been at the center of low back pain (LBP) management for decades. Yet LBP remains the leading cause of disability worldwide. So should we stop promoting these messages?
Everybody has heard this advice: “Be careful when you lift,” “try to keep your back straight.” These messages have been at the center of low back pain (LBP) management for decades. Yet LBP remains the leading cause of disability worldwide. So should we stop promoting these messages?
If you are short of time, the simple answer is YES. Here are some reasons why.
Spinal flexion is not dangerous for the back
Observational studies have not identified spinal flexion as a risk factor for LBP. Recent biomechanical studies (see here and here) have also shown that joint forces and loads at the lumbar spine are similar during stoop lifting (lifting with a flexed spine) and squat lifting (lifting with a straight back and knee flexion), and that we have more strength when lifting with a flexed spine. This means that lifting with a flexed spine won’t damage your back!
People with LBP already move with a straight back
Multiple studies have shown that people with LBP move slower (less angular velocity), with less lumbar flexion (a straighter back), more knee flexion, and with higher levels of trunk muscle activity. Thus, people with LBP already tend to move with a straight and stiff back! Other studies have also shown that people with LBP move in a more rigid manner in other daily-life activities, such as sit-to-stand, stepping-up, and gait. So why reinforce this pattern?
Protecting your back and reducing spinal flexion is not helpful
For many years, the promotion of lifting with a straight back through manual handling advice has been very popular. However, a Cochrane review demonstrated that manual handling advice is not effective in preventing and treating LBP. This means that being careful about how you lift will not help you manage or prevent LBP.
Now that you understand why these messages are not helpful in preventing or treating LBP, I would add that they can also have negative consequences. In fact, these messages have been so widely promoted that they are embedded in Western culture and widespread in the general population. These messages foster beliefs that the back is vulnerable and needs to be protected. This might lead to the development of fear and avoidance behaviors, which have been strongly associated with LBP disability.
So what?
It is time to change the narrative about the back.
There is a need to promote positive messages about the back, reinforcing that it is strong, adapts well to load, and that we have different options for moving. Healthcare professionals should routinely discuss this information with patients suffering from LBP, and mass media campaigns are needed to reach a large part of the general population. These strategies should be supported by public authorities and leaders in the field, as we saw recently in the a call for action in the Lancet series on low back pain.
Guillaume Christe, PhD student, Haute École de Santé Vaud (HESAV), Switzerland.
The BonePain Patient Ambassador Group
Conceptually, my first interest in patient advocacy began about 20 years ago, when my mom was struggling with an undiagnosed pain disorder and half of my family would comment, “It’s all in your head.” Hopefully, invisible conditions (i.e., conditions that are not immediately apparent to others) are not stigmatized quite as much anymore.
I grew up in a large town in the middle of the Italian Alps where, as you might expect, there are no specialist clinics. That meant it was two years and many failed treatments before my mom figured out that she has chronic regional pain syndrome. Once she knew, there was one thing that really helped her, aside from the right medication: THE INTERNET! Surfing the web back in the early 2000s (a time when the Internet speed was about 100K bits/sec, or 2,000 times slower than today), she connected with an online support group of people with her condition. Finding someone who actually understood her condition and shared similar daily life struggles made all the difference.
Fast-forward 15 years of not thinking about advocacy again (I was too busy watching cells under the microscope), and I found myself a PhD student at an EU-funded international training network. This was a partnership between universities and industry in which PhD students are trained on cutting-edge science. The focus of our group BonePain was to better understand – you guessed it – bone pain. **Spoiler Alert! More on this topic next week.**
One of the aims we set for our group was to increase awareness of those bone diseases where pain is a major symptom. Three of us ambitious students decided that we would change the world (a common theme for scientists) and bring research to patients; the BonePain Patient Ambassador Group was born. We decided to contact patients through already-existing advocacy groups and were utterly surprised that very few groups responded to our noble request. That was probably the first time we were brought back to Earth and realized how hard advocacy is. In the end, we managed to interview three patients.
After gaining informed consent to make those interviews public, our PhD, unfortunately, came to an end. To our relief, our advocacy efforts did not. In 2019 BonePainII was born, with five new PhD candidates joining the Patient Ambassador Group. Together we developed a new concept for the group: to intensify the exchange between science and patients by directly involving them.
If you are a patient with bone pain and are interested in co-creating a solution to bridge the gap between science and patients, feel free to get in touch with us to shape the future of the BonePainII Patient Ambassador Group. To learn more and keep up-to-date with our work, follow us on Twitter @pagbonepain.
Larissa de Clauser, PhD from University College London, now based in Italy.
Are You With Joe Fox or Sheldon Cooper?
I’ve had the good fortune of studying, working and living in five countries with rich heritages, cultures, and amazing food! This global citizenship has allowed me to meet some amazing people and have extraordinary experiences. As a researcher, it’s second nature to think of these experiences from a clinical research lens.
From 2010-2012, I shared my home with three other amazing young professionals, all from different countries and with different training. We had a tradition of having a meal and watching a movie together at least once a week. We invited friends (new and old) to come along. On one such occasion we watched the movie You’ve Got Mail. About an hour into the movie there is a scene where Joe Fox (played by Tom Hanks) brings flowers to Kathleen Kelly (played by Meg Ryan), who is down with a cold, and makes tea for her. I noticed during this scene that the room went quiet, everyone with a gentle smile on their face. We were all in agreement that it was ideal partner behavior! He showed love and care for her, effortlessly. It brought warm fuzzy feelings to our hearts.
On another occasion, we ended up watching The Big Bang Theory episode where Amy Fowler (played by Mayim Bialik), also down with a cold, gets a visit from her boyfriend Sheldon Cooper (played by Jim Parsons). Sheldon makes Amy a hot beverage, sits with her, and empathizes with her in his own way as he has been taught by his mom and meemaw (grandma). But as his bedtime nears, he gets up to leave. Amy is shocked. She can’t believe Sheldon wants to leave. This time the room was in an uproar. Opinions were divided. Some supported Sheldon’s choice to prioritize his own sleep and self-care, while others thought he was being insensitive.
As a researcher interested in “dyadic coping,” I’m intrigued by these experiences. Dyadic coping is the coping that transpires between intimate partners when challenged by a common stressor, such as one partner’s illness. Unlike other forms of social support, dyadic coping is not altruistic in nature in that it is intended not only to support the unwell partner but also to maintain harmony in the relationship.
The best evidence available indicates that partners who provide extended protective caring behaviors (taking on chores, e.g.) reinforce disability and lower patients’ confidence in functioning well when in pain (self-efficacy). While it may seem natural for partners to provide protective caring in the immediate period, if these responses continue indefinitely, they increase the risk of nonrecovery in patients.
A study conducted during my Endeavor Fellowship in 2018 under the mentorship of Professor Liesbet Goubert showed that, compared to a partner’s report of the dyadic coping they provided, the chronic pain patient’s own perception of the dyadic coping they received is a better predictor of both the patient’s and partner’s relationship quality and psychological outcomes over time.
Interestingly, we also observed that negative dyadic coping (for instance, when the partner withdraws from the patient when the latter is in pain) exhibited stronger effects over time on the same outcomes mentioned above for both patient and partner, compared to supportive dyadic coping.
These findings show the importance of appropriate appraisal of partners’ expectations regarding dyadic coping for enhanced overall well-being, for both the patient and their partner. However, we need more research to clarify if these findings vary depending on other contextual factors such as sex, gender roles, and sexual orientation.
In light of these findings, if we revisit the two scenes described above, both Joe and Sheldon seemingly displayed similar caring actions, visiting the ill partner, making tea, and spending time with them. However, there was a difference in Kathleen’s and Amy’s interpretation of these actions. Was this because Joe’s caring actions aligned with Kathleen’s expectations, whereas Sheldon’s did not align with Amy’s expectations? What do you think? Let me know @DrManasiMurthy.
Manasi M Mittinty, lecturer, University of Sydney, Australia.
“Outcome Measures Measure Outcomes – Right?”
Imagine you are a clinician and have recently been treating a patient with low back pain. After three weeks of treatment, the patient’s low back pain has gone down from a 7 to a 2, on a 0-10 pain scale (0 = no pain, 10 = worst imaginable pain). The patient is experiencing less pain and you are feeling pretty good about the outcome – you did it! But did you, really?
The problem with the above scenario is that the patient’s reduction in pain may not be because of the treatment you provided. When I first transitioned from clinical practice to PhD research, a colleague shared a paper with me that completely changed the way I viewed treatment outcomes. The paper highlights the problem with interpreting changes in outcome measures to justify the success of treatments. “Outcome measures measure outcomes. They do not measure the effects of treatments.” In fact, the effects of treatments and changes in outcomes are very different things.
So what’s going on here? The change we saw in the patient’s low back pain might have been due to the treatment we provided, but it might also have been due to forces outside the treatment. These “non-specific effects” include natural history of the condition (many conditions tend to resolve on their own over time); regression to the mean (many conditions are episodic and fluctuate, with people commonly seeking care when the condition is at its worst); and placebo effects (famously, expectation and classical conditioning). This paper provides a nice overview.
If we are interested in understanding the effects of treatments, we must look beyond the change in outcome from a single patient (clinical change over time), to well-conducted randomized controlled trials that compare the average outcome of many patients who either receive the treatment or do not.
Outcome measures have their place in clinical practice, but we must remain mindful that they measure outcomes, not treatment effects.
Aidan G. Cashin, PhD candidate, Neuroscience Research Australia (NeuRA), University of New South Wales, Australia.
Orofacial Pain – From the Bench to the Dental Chair: An Integrated Experience
Orofacial pain affects facial structures, impacting quality of life, since we depend on those structures for drinking, eating, and verbal and nonverbal social interactions. Treating this condition is challenging. Drugs like opioids, indomethacin, ibuprofen, and corticosteroids have several side effects over the long term. Knowing that orofacial pain can last for years, the search is on for safer alternatives. Researchers are looking back to our first source of medicines, nature, to discover new pain killers and anti-inflammatory drugs.
But how can plants be turned into medicines to ease pain? How are these treatments developed for people with orofacial pain?
This was something I pursued as an undergraduate researcher in dental school. In the laboratory we tested natural products, isolated from plants or algae, in rats with orofacial pain. Then we sent the raw extracts to chemists who isolated their major constituents. Each isolated component was then tested and compared to standard treatments, such as indomethacin or morphine. Alternatively, chemists modified the isolated molecules to see whether this enhanced their therapeutic properties. We also assessed the toxicity of these substances in animals, as safety was also an important issue to address. All in all, it was wondrous teamwork.
While I enjoyed that work, I am well aware that testing these substances in animals is just the beginning of a long journey when it comes to designing a new drug. From the discovery of a molecule with analgesic properties in rodents to its use in humans, more than a decade is necessary to ensure safety and efficacy in different pain conditions. Considering the side effects that limit the use of current medications for orofacial and other types of pain, the need for alternative treatments justifies the financial and time investment in basic and clinical research.
My supervised clinical appointments with patients while I was in dental school made me understand how debilitating pain can be. Seeing people suffer is different than reading pain definitions in textbooks. That no drug therapy gives full pain relief made me aware of my role as both a healthcare professional and a pain researcher; the importance of understanding pain and how to treat it encouraged me to work on basic mechanisms of orofacial pain.
To learn more about orofacial pain classification, see herehere and herehere, here, and here for more on the use of natural products to reduce orofacial pain. Some of these studies were performed by the group in which I worked previously.
Francisco Isaac Fernandes Gomes, DDS, PhD student, University of São Paulo, Brazil.
Understanding the Failure of Translation in the Pain Field
The ongoing war on opioids brought on by the opioid and addiction epidemic, where half of all overdose deaths in the US are related to prescription painkillers, unfortunately tends to hurt the people who need pain medications the most: chronic pain patients.
As both a chronic pain patient and a pain researcher, I understand the frustrations coming from both sides.
Unfortunately, I fall in the category of pain patients with what is referred to as intractable pain, meaning complex and constant pain that is difficult, if not impossible, to manage with standard medications. On my good days, to function even slightly as a member of society, I am forced to rely on opioids because of a lack of alternative medical treatment options.
On my bad days, getting out of bed and moving to the couch feels akin to climbing Mt. Everest. Acting as a safety harness, opioids help shrink down that trek from a towering mountain into an indoor rock-climbing wall; it is still difficult, but much less daunting. Even though opioids significantly help me cope with my pain, there is never a moment that I am not in the clutches of the relentless monster that is chronic pain.
Pain researchers and drug developers are familiar with the issues relating to drug translation failures in the pain field. However, patients suffering from pain and the broader public do not typically have access to the insight about why it is so difficult to come up with new, more effective drugs than what is already available. Only 10% of newly discovered drugs that have been confirmed to work in rodents reach approval for human use by the FDA. For new pain therapeutics, that success rate drops down even lower, to a staggering 2%.
I have experienced firsthand just how draining and all-consuming the chronic pain monster can be. It is hard to keep a positive outlook when constantly being bombarded by messages from the media, family members, and even doctors that I am in the wrong for using opioids when opioids are the one thing that gives me some semblance of relief. I should not have to feel guilty about or constantly defend taking a medication when all other options have failed. Even more so, I should not have to constantly be in fear of having access to pain medication taken away from me because of tightened regulations brought on by the DEA – a very real situation occurring daily for pain patients.
Therefore, I strongly believe it is of utmost importance to communicate the current problems preventing new pain medications from reaching human use as well as what pain researchers are doing to correct those problems. This way, maybe I can offer a glimmer of hope to pain patients, and to the public, by bringing awareness that, while it may not feel like it, progress is being made to identify new pain treatment options.
Here is a video I created where I talk about my current research exploring some of the translational issues within pain research.
Sarah D'Angelo, undergraduate student, Rutgers University, US.
Is My Pain Gone, or Am I Just Hungry?
Have you ever been in so much pain that you don’t feel like eating? But after hours and hours of not eating, you start getting really hungry and somehow muster up the energy to get up and eat? I know I have. Aside from getting the sarcastic, “Now you’re not in pain anymore? See? It wasn’t that painful after all” from my siblings as they watched me find my way to the fridge, I never understood how for this very brief moment, my pain went to the back of my mind, and shortly after eating, it all came back.
I am happy to report to all my family members that, indeed the pain was bad, but perhaps my hunger was making my brain prioritize my need for survival by blocking the sensation of pain. In a study published in 2018, Amber Alhadeff and colleagues investigated the interplay between hunger and pain. The major finding of the study was that hunger serves as a pain reliever, or analgesic, much like ibuprofen. Using an inflammatory pain model, the authors compared mice that had been eating whenever they wanted to mice that had been deprived of food for 24 hours.
One way to look at pain in mice is to observe the amount of time they spend licking the inflamed paw; the more they lick, the more pain the mouse is in. Alhadeff and colleagues found that the mice spent only a third of the time licking their inflamed paw when they were hungry, compared to when they were well fed.
What’s also important to note is that this analgesic effect of hunger did not only affect licking behavior but also the emotional state of the mouse that would otherwise be in pain. When mice are trained to associate a certain location with pain, they usually will avoid that location in the future. However, mice that were hungry during the training period did not later on avoid a location that had been associated with inflammatory pain, behavior suggesting that they did not find the inflammatory pain experience aversive during training. In addition, hungry mice had increased locomotor activity (they moved more), much like I did when I eventually got hungry and walked faster towards the fridge than I did to the bathroom.
This does not mean the solution to resolving pain is for us to starve ourselves (please don’t). But this is exciting because in addition to these behavioral observations, the scientists who led the study were able to identify molecular features in the brain that were responsible for this hunger-driven analgesia. In this case, neurons activated during hunger directly signal to pain-processing neurons in a region of the brain called the parabrachial nucleus and block their activity. This sheds light on a new way to control how we perceive pain and a potential new therapeutic avenue for pain.
Sara Hakim, PhD student, Harvard Medical School, Boston, US.
When we discuss pain, we often understate its importance in protecting us. Pain is described by the IASP as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.” A note accompanying the revised definition acknowledges that pain has an adaptive role. But often there is still not enough emphasis that pain is an absolute necessity.
As pain researchers, our efforts to understand the mechanisms and produce treatments for pain may give the impression that life would be better if we did not experience pain. But would it? Pain is a major reason people seek medical help both for themselves and their pets. It alerts us to other problems that may otherwise go unnoticed, such as an infection on one’s back. This could become a much more dangerous problem if not for the initial instance of pain that prompts checking of the affected area.
So what happens if we cannot feel pain? This question can be answered by a rare disorder known as congenital (from birth) insensitivity to pain (CIP). There are only around 300 cases worldwide, and it is characterized by a loss-of-function mutation in NaV1.7, a particularly important sodium channel in nociceptive signaling. The result is no painful feeling at all. Patients with CIP do not have the fear of hurting themselves like others do. It is very difficult to explain to a child with CIP, “Be careful; you may hurt yourself,” as this is not something that impacts them. Indeed, case studies have noted children breaking their legs because of repeatedly jumping down stairs, since the unpleasant sensation from doing so once is not there to stop them from doing it again.
Some of the potential consequences of the condition are almost impossible to mitigate. Have you ever bitten your tongue? It is enough to make your eyes water and immediately stop biting, yet it is common for people with CIP to bite off chunks of their tongue when eating or chewing in general. It is difficult to prevent something you cannot see or feel; even tips of fingers and thumbs can be lost this way. Though difficult to comprehend, this does help me to think more about the very normal things that would be a constant threat to well-being and render this condition very debilitating, both for people with CIP and for those caring for them.
There are some further interesting symptoms of CIP that have been very useful in identifying normal bodily processes that require nociceptors to function normally. One of the biggest threats to those with CIP is normal changes in temperature. Pain is important in identifying extremes in temperature – to prevent burns, for example. Just as important is sensing smaller changes in temperature; this is also the role of some nociceptive neurons and closely related neurons. In CIP the body is unable to accurately sense changes in temperature, often failing to trigger a sweating response and other usual responses to a normal increase in temperature, such as being out in the sun.
Conversely, a person with CIP may fail to shiver or redirect blood as would occur in cold weather. This is in addition to the person being unable to recognize that it is uncomfortably hot or cold and wear appropriate clothing, for example; anyone who has tried to put a coat on a child will appreciate that this is far more difficult if that child does not get uncomfortably hot or cold. This poses a significant threat for people of any age with CIP, as they do not have the same physiological or behavioral response to changes in temperature, and so are at risk of heat stroke, burns, or hypothermia.
The message that I take away from this is how the loss of one sensation, a sensation we generally don’t particularly like, can impact the most basic aspects of daily life and make the things we don’t ever have to think about suddenly be in the forefront of one’s mind. It can be interesting to take a moment to think of examples of how a lack of pain might affect the things you do. It can often provide some small insight into the complexity of the human body and a complex condition such as CIP.
For anyone wishing to read more there are some excellent articles on the science and personal aspects of CIP across the PRF, RELIEF, and PAIN websites. Do check them out here, here, and here.
Frederick Jones, CASE PhD student, University of Leeds UK and Eli Lilly & Co, US.
A Dynamic Life With Freedom, Good Relationships, and a Sense of Community Belonging Makes Me Happy. What Does Happiness Look Like to You?
I was once told by a primary headache patient that the depression starts after the attacks – joy disappears and sadness kicks in. We know that migraine can increase the risk of depression. It is also no surprise that the presence of pain and depression symptoms have been negatively correlated with happiness. So what can headache patients do to get back on their feet after an attack? If experiencing depression or having depressive thoughts, it might be worthwhile to shift focus to finding the answer to the question, “What makes me happy?” and then implementing it into daily routines. At least this is what my mom does after her migraine and cluster headache attacks.
My mom created her own “happiness list,” which includes being in the sun, spending time in nature, going for walks, spending time with loved ones, and doing yoga and meditation. What would a “happiness list” look like for you or your patients? Happiness for me is also about living in a society where people are friendly and help each other; having a sense of community belonging matters to me. Previous studies have revealed an association between sense of community belonging and self-perceived health such as mental health (see here and here). Interestingly, the odds of major depressive disorders decrease with increasing levels of a sense of community belonging. Even so, this outcome might be different for primary headache patients, as the pathophysiological mechanisms, to some degree, differ from those underlying major depressive disorders, but everything is worth considering.
What makes people happy on a global scale? As a Dane, it quickly caught my attention that Denmark rates as one of the happiest countries in the world. “Pretty cool,” I thought. My research mind took over one day, and I found myself looking closer at the World Happiness report, inspecting the results that led to this conclusion. I found that factors such as generosity, freedom to make life choices, and healthy life expectancy were considered in the happiness ranking. I appreciate the freedom we have in Denmark. One example I would like to share is that in most Danish research environments that I know of, students have a strong voice – their opinions and ideas are valued. Freedom to choose and being given a voice are aspects that make me happy in my professional life.
Happiness can be found in different corners of life ranging from healthy relationships and lifestyle choices to exciting adventures and, for some, career progress – all of which often relate to some form of freedom. Not all days are filled with happiness, but you can do a whole lot to tilt the balance towards increased positive affect and decreased negative affect; by affect I mean “the collective term for describing feeling states like emotions and moods” (see here and here). Another facet to keep in mind is that depression has been described as a “disorder of affect dysregulation.” So how can you and your patients tilt the balance in their favor? Maybe creation and implementation of individualized positive affect strategies to improve happiness would do.

Simona Denise Frederiksen, postdoctoral associate, University of Calgary, Canada.
You can probably guess that the answer is yes – otherwise I would not be writing this blog post. But it’s not something that easily comes to one’s mind. If we see scars on somebody, we usually sympathize with them about their appearance, about what they might’ve been through when they got those scars. We all think of scars as remnants of past pains, but in truth, scar pain can be just as bad as the pain of the original injury – or even worse!
So, what exactly makes scars painful? To answer that we first need to understand how scar tissue is different from healthy skin. The first thing that happens when we injure ourselves is inflammation –a sign of the body's immune system kicking in to protect the wound from infection. The immune cells produce certain molecules that can activate fibroblasts – a type of cell that resides in the mid-layer of the skin and produces collagen (a kind of tough, white protein fiber) to reconnect the broken tissue. In scar tissue, the collagen proteins grow in a single direction rather than in a multidirectional pattern as they do in healthy skin. This structure makes the scar tissue tougher and less flexible, which causes skin tightness. This can result in pain, and it makes it more difficult to move freely.
Another way in which scars can become painful is when the body grows an excessive amount of scar tissue – a process called fibrosis, which happens when fibroblasts do not clear over time and cause long-lasting inflammation. And in other cases, when the original wound was deep and damaged the nerves in the skin, neuropathic pain develops, which causes long-term altered sensation in the scar area, such as sharp, shooting, or burning pain; itching; and paraesthesia – like the "pins and needles" feeling you get when you sleep with your arm under you.
The reason why I’m telling you about this is because recently I joined a lab that tries to understand what exactly happens in one particular type of scars called keloids. These are enlarged, raised scars that spread beyond the boundaries of the original wound – they’re quite eye-catching, and sometimes painful and most often very itchy! And the worst thing is that they can happen from very minor things like acne and piercing in people who are predisposed to developing keloids. I got the chance to survey some people suffering from keloid scars, and I was quite touched by what they had to say, especially about the lack of awareness around pain in scars: “People don’t understand that scars can be painful!!!”
Other people told me that they’re extremely self-conscious when their scars start itching when they are in public and they have the urge to scratch in unusual places. These people don’t qualify as chronic pain sufferers because usually if you manage to get rid of the scar, the pain goes away. For this reason, pain and itch management in keloids is quite neglected. But there’s a catch – people can always get other scars from the most minor injuries!
So, I’ve done my part by letting you know that scars can be painful, and we can all do our part by being more mindful next time we see someone scratching in weird places at the supermarket.
Denis Duagi, PhD student, King’s College London, UK.
Week 1: Tuesday, March 9, 2021
It’s a Small World – An Intricate and Complex Small World
A Video Introduction: Oakley Morgan
Is This All About Communication?
A Researcher, a Pain Patient, and an Artist
From Patients to Molecules: My Journey to Pain Research
The Pain of Climbing Spirit Animals
A Career as a Scientist: Not a Cross to Bear Alone
The Paths That Brought Me Here
On the Lookout for Answers to Solve the Pain Epidemic: We Are in This Together!
Harnessing the Power of Human Connection to Manage Pain
A Focus on Spinal Cord Injury Pain
It’s a Small World – An Intricate and Complex Small World
Hello PRF readers! My name is Sara Hakim, and I am a PhD student at Harvard Medical School studying peripheral neuroimmune interactions in diabetic neuropathy.
I did not know this career path existed until I was 20 years old. I grew up in the heart of Cairo, Egypt, and was always interested in molecular and cellular biology, thinking that meant I was destined to become a pharmacist. Following the Arab Spring, my family and I moved to the US, where I started my college career, and my excitement about research was ignited.
I remember sitting in my molecular biology course fascinated by the complexity of the process of transcription and the fact that this is happening right now, in my skin, and I cannot even see it. I recall wondering how such a tiny cell knows to perform such intricate processes, and imagining the helicase coming in and snapping open that DNA, the transcriptase lodging in, and the little nucleotides floating around waiting for their turn to be annealed. It was my very own little Disneyland ride, where so much is going on and “It’s a Small World” is playing in the background.
When I finally started my first research experience, I was drawn to the even more elaborate complexity of the nervous and immune systems. Not only is so much going on within the cells, but the cells are actually able to communicate with each other in their own “language” and coordinate behavioral and physiological responses. This informed my excitement about the study of neuroimmune interactions, especially in the peripheral nervous system.
Conceptually, the sensory nervous system and the innate immune system serve very similar roles: to detect and protect us against danger by virtue of interacting with the outside environment, recognizing clues of danger, and keeping a memory of those stimuli. Whether the stimulus is a bacterium, a noxious temperature, or a foul smell, our body has these systems to recognize that these are “bad” stimuli and to avoid or fight those dangers.
At the start of my PhD, I wanted to study a pain condition that is prevalent in today’s population. I chose to study diabetic neuropathy because of the alarmingly increasing rates of diabetes that I believe will make diabetic neuropathy one of the most prevalent painful conditions in our society in a matter of decades. In addition, there is a large body of work looking at immune system dysfunction in diabetes and obesity that could explain many still-unexplained facets of diabetic neuropathy. I hope that throughout my PhD, I can work towards a better understanding of how dysfunction of the immune system could be contributing to diabetic neuropathy, and that my work from here onward could add to our overall effort to understand and treat painful conditions.
I am thrilled to be part of the PRF Correspondents Program, and look forward to sharing my thoughts with the PRF community and learning from my fellow Correspondents!
Sara Hakim, PhD student, Harvard Medical School, Boston, US.
A Video Introduction: Oakley Morgan
Oakley Morgan, PhD student, University College London, UK.
Is This All About Communication?
The difference between the public and the scientific community in understanding pain has always struck me. The knowledge gained by brilliant researchers over the past few decades is still struggling to reach patients, the general population, and even many healthcare professionals. This is a major issue, as it hinders the implementation of best practices.
This is why communication and education on current knowledge about pain should be one of our main priorities, along with the advancement of knowledge in this field. What is the point of knowing so much if this knowledge does not ultimately reach its main target –people suffering from pain?
I am Guillaume Christe, a physiotherapist from the French-speaking side of Switzerland. I live with my wife and two daughters in a small town near Leman Lake and the Alps. I share my time among teaching physiotherapy students, working with patients in a private clinic, and doing my doctorate at the Swiss BioMotion Lab, when I am not skiing or eating a cheese fondue.
Communication is at the heart of my professional life. I try to reach a shared understanding with patients about their problems and to help them find the best management solutions. I try to help physiotherapy students become educated, efficient, and caring health professionals. I try to precisely interpret and communicate my research findings. After almost 15 years since I graduated as a physiotherapist, I can’t find a more important skill than communication, and I am still struggling with it.
My main area of interest is low back pain. I am particularly curious about how people with low back pain move, and how movement is associated with pain, disability, and various emotional and cognitive factors. I am also very interested in people's beliefs about low back pain, how these beliefs influence their behavior and decisions, and how we can help them to have more helpful beliefs about their condition.
I am really happy to be part of the PRF Correspondents Program and am looking forward to the coming weeks!
Guillaume Christe, PhD student, Haute École de Santé Vaud (HESAV), Switzerland.
I was born in Germany’s westernmost city, Aachen, known for its splendid cathedral and long Roman history. I then moved to Maastricht in the Netherlands, the city where the Maastricht Treaty was signed in 1992, laying the foundation for what is now the European Union. Maastricht is still my home now. Here I studied psychology, and later did a research master’s degree in psychopathology. It was also here, while studying statistics of all things, that I met the woman who would later become my wife. What are the odds? For my PhD I again crossed another border, to Leuven in Belgium, home to Belgium’s oldest and largest university. Fittingly, I took a museum piece of a train almost daily to get to my office in Leuven. To some, this might seem like a truly cosmopolitan and exotic story, but be aware that all these cities are just about a 60-minute drive from one another. If anything, I suppose it is a truly European story.
I started out treating untreatable psychopaths in a forensic hospital in the Netherlands, and my fascination for forensic psychopathology and why people can do horrible things to one another has stayed with me to this day. This work demonstrated so clearly that an individual’s story is really always also the story of their social and cultural circumstances.
No man is an island.
Where we grow up, who we interact with, the friendships and families that we fall into, for better or worse. The social networks we find ourselves in, always, and how they can drastically change the course of our lives. Social psychology, then, seemed quite naturally to be another area that I found myself drawn to.
By stroke of luck and chance, like anything in life, I started a PhD project in a group that lives and breathes the psychology of pain, more specifically the role of fear and avoidance in the development of chronic pain. Under the supervision of Prof. Johan Vlaeyen, Dr. Ann Meulders, and Prof. Liesbet Goubert, I was fortunate to get the freedom to develop my own project in this new field. Pain was said to be biopsychosocial, and yet it seemed to be more BIOpsychosocial in the academic literature and practice. So, going back to my earlier roots, I brought what I knew and loved to the field of pain: I studied how our social surroundings, especially social interactions we experience as threatening, shape our experience of pain and how we communicate pain to others.
I found my academic home at this intersection of social, health, forensics, and learning psychology. I still investigate how all our pain experiences, all experiences really, are also always social. How they are shared and impacted by the presence of others. I see it in my two-year-old son, who keenly observes his mom and dad when they get hurt, learns from it, and subsequently tells us about the painful experiences he had and how they made him feel, with an accuracy and adeptness that is baffling to us. I see it in the patients with chronic pain, who tell us that sometimes the most painful thing about their affliction is that others will not take it seriously or that they are excluded because of it.
No person is an island. I do believe that once we embrace this, there is a lot of hope to be found here.
Kai Karos, PhD, Centre for the Psychology of Learning and Experimental Psychopathology, KU Leuven, Belgium.
A Researcher, a Pain Patient, and an Artist
Hello PRF readers!
My name is Sarah D'Angelo, and I am graduating with a bachelor’s degree in biology, and a minor in psychology, this May 2021 from Rutgers University in Camden, New Jersey. Currently, I am interviewing for programs to start a PhD studying the biopsychosocial aspects of pain in the fall 2021. I was born in Texas and am currently residing in South Jersey – just over the bridge from Philadelphia.
I have always been fascinated by the inner workings of the mind, and knew from a young age that my calling was to earn a doctorate in neuroscience to pursue a career in research. At first, I was unsure of what I wanted to specialize in, but I knew that it would somehow involve the brain.
As I began to grow as a young academic and scientist, so too did my pain. The minor aches evolved from being a slight inconvenience to daily all-encompassing, widespread pain impacting every aspect of my life. So I first entered the realm of pain communication through the lens of being a pain patient myself.
For me, learning to cope with my pain meant diving headfirst into the life-long journey of becoming as knowledgeable as possible in all things pain. Gaining insight into the mechanistic and neural underpinnings of the subjective perception of pain validated my own experience in a way no doctor or medication could. The more I tried to understand my own pain, the more I realized how much is still unknown. I became increasingly captivated by the field in its entirety; I knew I had found my niche.
As an undergraduate, I am leading a meta-analysis of the language and behavioral techniques utilized in rodent pain studies to explore how the way we talk about pain as scientists in the preclinical setting may be adding to the barriers preventing new pain medications from being successful.
Pain is often dismissed by society as being “all in your head.” Facing the frustrations brought on by the isolating nature of pain fuels my passion for bringing visibility to chronic pain, as an advocate not only for myself but a voice for pain patients who may not have the resources to do so on their own.
By openly talking about my own struggles in navigating life as a pain patient in everyday life and throughout my journey as a scientist, I hope to help break down the stigma associated with having chronic pain.
One of my favorite things to do is find innovative ways to merge the worlds of art and science as a means to distill down complicated subjects. Making science more accessible is a driving force for my continuous experimentation with various illustrative and narrative conceptualizations of pain-related topics for communication with the general public, patients, and my peers. See a few examples of my pain-related art below.
I am excited to have the opportunity to enhance my personal development as an early-career pain investigator and science communicator as a PRF Virtual Correspondent.

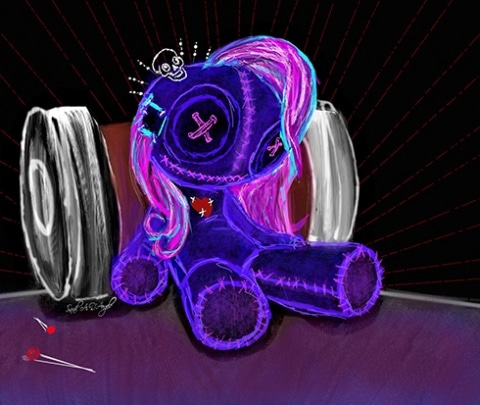
Sarah D'Angelo, undergraduate student, Rutgers University, US.
From Patients to Molecules: My Journey to Pain Research
Hello PRF world! I’m very excited to take part in the Correspondents program, especially since I’m just beginning my journey into postgraduate research. Even though I haven’t even started my PhD yet, my journey in pain research has been quite long and varied.
My first contact with pain research was in my first year of my undergraduate studies – we had to do the McGill Pain Questionnaire on a friend as an assignment. Now, in hindsight, I probably did it all wrong, but I remember being so fascinated by all the words you can use to describe pain – it was definitely a vocabulary-enriching experience for my foreign university fresher self who struggled to even understand British people when they spoke. In my second year I realized how drawn I was to pain research. There are so many dimensions to it: You can approach it from a sensory biology perspective, from a cognitive perspective – and that is just the physiological function of pain. Things get even more complex when you look at the pathology of pain disorders.
Persistent pain was a bit of a foreign concept to me, I must admit – NSAIDs always work for me – so I decided that I wanted to first understand what chronic pain sufferers are going through. I did a summer placement in clinical pain research, working with children with neuropathic pain, and that was when it dawned on me just how terrible these disorders are. Can you imagine being a seven-year-old who might just spend the rest of life in pain?
I then wanted to use my training as a neuroscientist to understand more about pain – we all know that pain is in the brain, right? After thoroughly examining all the ways you can investigate pain in the brain for my dissertation, I went on and did my master’s project on neural circuits for pain and protective behaviors. Although I really enjoyed basic research, and my fascination for circuit neuroscience remains deeply embedded in my identity as a neuroscientist, I decided that I wanted to pursue more translational research – and here I am on a DTP (Doctoral Training Partnership) at King’s College London, an institute that’s full of translational pain researchers. Since the start of this academic year I’ve modeled the circuitry for headache pain in a dish, engaged with patients suffering from keloid scar pain, investigated fibroblast-to-neuron communication in fibrosis and pain, and will soon start a project looking at fibromyalgia – a very controversial pain disorder with both neurological and immune components.
Being part of the PRF Correspondents Program, I will get to dive deeper into various pain research fields, and I hope it will help me decide on a PhD project that will reach PRF in a few years’ time! I am very excited and grateful to find myself on this journey, and hope to learn and share inspiring research with you over the next couple of months.
Denis Duagi, PhD student, King’s College London, UK.
The Pain of Climbing Spirit Animals
Sometimes a seemingly ridiculous conversation can give you some deep insights into yourself. I had this Eureka moment last week, when I was asked what my spirit animal is. Intuitively, I selected the naked mole rat, simply because in terms of pain perception, it is the most intriguing mammal we have come across so far. To be fair, I have nothing in common with the naked mole rat, aside from being pretty blind without glasses. The next day, spring was in the air, and I landed on a rock-climbing wall. There, it hit me! Heating up on a rock, agile vertical movements, I am Larissa the lizard!
Unlike most lizards, I have also ventured on winter climbing sessions with freezing winds in the UK’s well-known Peak District. And this is when you get a full-body experience of pain perception (and inhibition), while climbing well above your usual level. During winter, our hands remain too cold for sweating, resulting in a better grip on the rock, thanks to increased friction. On the downside, if hands are too cold, they will get numb! While normally a painful experience, during climbing our focus is 100% on not falling off the rock. In addition, the brain is on fire, releasing dopamine, norepinephrine, serotonin, and endorphins in several areas involved in pain processing. As a consequence, you completely ignore your numb and painful hands.
My climbing experience has probably given you a good insight into the complexity of pain, a topic that has fascinated many of us and brought me to join the lab of John Wood at University College London as a PhD student in 2015. Having grown up with my mom developing chronic regional pain syndrome, I wanted to understand what causes chronic pain. Being a molecular biologist by training, for me all the answers lie in a targetable molecule. Although I have always been fascinated by the intricate workings of the brain, I immediately recognized the peripheral nervous system as the site of action for therapeutic intervention.
So I started the roller coaster journey that is the PhD, to investigate peripheral mechanisms of cancer-induced bone pain. Three papers and a thesis later I joined the lab of Sarah Flatters at King’s College London and worked on the identification of peripheral targets in chemotherapy-induced neuropathic pain. My future plan is to join Eurac (South Tyrol, Italy) as a postdoc. There, I want to use sensory neurons derived from human induced pluripotent stem cells to study the effects of mutations in a potassium channel which are associated with a pain phenotype in humans. Ultimately, my goal is to identify new treatment options for these people using a translational model.
I am more than delighted to have been selected for the PRF Virtual Correspondents Program, which will be a great opportunity to engage with the scientific community and the general public interested in pain research. In this regard, I am looking forward to telling you all about the Bone Pain Patient Ambassador group in the coming weeks….
Larissa de Clauser, PhD from University College London, now based in Italy.
Through many well-conducted clinical trials and systematic reviews, the pain research community has developed an evidence base of helpful treatments for people with pain. As a practicing exercise physiologist, I routinely provided some of these evidence-based treatments to my patients. Although people recovered to varying degrees, one question always kept bothering me: “It seems to help, but why?”
This question persisted, and by good fortune I was able to undertake a PhD at Neuroscience Research Australia (NeuRA) to start asking why. Currently, there is a lack of well-conducted research investigating why common treatments work. This is partly because most clinical trials take a “black box” approach without considering the precise mechanisms of treatments. My PhD research begins to address this knowledge gap by using formal methods to investigate treatment mechanisms – mediation analysis. Information gained from this line of research can help improve the effectiveness of treatments and assist with their implementation in clinical practice.
I look forward to contributing to the PRF Virtual Correspondents Program and sharing my research, ideas, and experiences I have gained negotiating the research landscape as a clinician-scientist in training. Research communication should always be transparent and open, but why not also be a little bit of fun? So let’s see how we go….
Aidan Cashin, PhD candidate, Neuroscience Research Australia (NeuRA), University of New South Wales, Australia.
A Career as a Scientist: Not a Cross to Bear Alone
Hi, everyone. My name is Fred, and I am a final-year PhD student with a collaborative industrial partner (a CASE PhD studentship) researching ion channels and pain. I thought I would introduce myself by sharing my journey up to now.
Back when I had slightly less of a beard and I accepted I was clearly not going to be a professional athlete, I started to think about the career I wanted to pursue. I had a particular interest in the brain and in solving problems, which led to two major callings: be a neuroscientist or train as a bomb disposal technician. Needless to say, it would have been smart to choose the “far safer” option and join the Army (joking, of course), but instead I followed the path of the weird and wonderful, and began this strange career where I try to make my brain understand how my brain works.
In starting to write this post, I realized that the story of how I ended up six months from handing in my PhD thesis and wanting to start exploring science communications is a story less about me and more about the people I have met along the way.
I don’t remember what drove me to apply to study neuroscience in the first place, but I do remember my first day at the University of Leeds. I had an introduction to the subject from an eccentric Scottish professor whose passion for neuroscience blew me away. I met a different academic who came to chat with my family and me when we were feeling extremely out of our depth, having never been to a large university or spoken to professors before.
During my undergraduate degree, I had lab members who taught me how to experiment and how to deal with the difficulty of generating results, despite having only been in the lab for eight weeks. The joke’s on them: I have been here an extra four years for my PhD.
There have been moments of unexpected generosity that I am very grateful for, such as the researcher I had never met who gave me a place to live (and giant cats to cuddle) when I started my CASE studentship at Eli Lilly. Also, the many other people at the company who took the time to teach me complex techniques and support me, despite the impending closure of the site.
I think about the group of people I battle with weekly on an American Football field in the windy north of England and the outlet that provided as a different form of pain to a PhD. I think about everyone who takes the time to ask about posters and talk to students like me at conferences; when this happens, there is a massive increase in confidence that comes with realizing people are interested in the work we have done.
The influence my supervisor, Professor Nikita Gamper, has had on me has been unparalleled during my PhD at the University of Leeds –not only for how to be a researcher, but also how to be a member of the pain and broader science community, to help, give back, and support others in the same way that others have supported me.
I have learned something from so many in our community, even in the briefest of encounters. Many of these people I would not hesitate to describe as friends.
So the story of how I came to be six months away from handing in my thesis and writing this blog post is actually a story of a lot of help from our amazing community. Nobody should go through science alone, and anyone who feels like they are alone: feel free to contact me.
Frederick Jones, CASE PhD student, University of Leeds, UK, and Eli Lilly & Co, US.
The Paths That Brought Me Here
Born in Sobral, a mid-sized city in the State of Ceará in northeast Brazil, I have always been fascinated by science. Not having chances to engage in scientific activities in primary and middle public schools, I always tried to get involved with related activities, for example, science fair projects and school newspapers. It was not until my second year in high school that I had the chance to join an immunology and biochemistry lab. While I mostly washed glasses and vials, I thought science was extraordinary.
My passion for science continued through the following years, and then in 2010 I was accepted to the dental school at the Federal University of Ceará – campus Sobral. Unlike several peers, I always adored the basic sciences. Three years later, I embarked on a life-changing experience of studying at the University of Sydney in Australia for 18 months. I gained a whole different perspective on life there. I experienced a new field, medicinal chemistry, which was fascinating. Being in the Science without Borders Program from 2013 to 2015 was hectic! Upon returning to Brazil in 2015, I applied to national and international grad schools. Being accepted to a few of the latter was quite shocking when I was still an undergrad, but I could only make my way to the former, which did not derail my dreams. Then, I moved to Ribeirão Preto in São Paulo in 2017, which was quite challenging – even more challenging than being 15,600 km away from home in the past.
Soon after kicking off a master’s degree in pharmacology at Ribeirao Preto Medical School at the University of São Paulo, my advisor exposed me to a new and emerging field to delve into for my master’s thesis: pain and metabolism. Being naïve to a field sometimes has its advantages, as the stumbling blocks along the way are usually unknown, so I promptly decided to accept his proposal. Since then, I have been working on metabolic changes of nociceptive neurons during chemotherapy-induced neuropathic pain. Understanding the bioenergetic profile of neurons, which I like to call “neuroenergetics,” is fascinating. Furthermore, I work on the role of metabolites that arise from bioenergetic imbalances in neurons and whether they could act as ligands, ultimately evoking intracellular signaling cascades in the dorsal root ganglia milieu. Being in this field is quite challenging!
Nonetheless, graduate school is not made only of (falling-apart) experiments. Taking active roles in science communication can also be of importance to us. Our society, regardless of our nationality, is fighting a pandemic of misinformation and misinterpretation that endangers science. Science communication should be encouraged as a way for scientists to fight the growing and global wave of fake news. Being an effective science communicator should ultimately be a “conditio sine qua non” toward a more socially engaged academia.
Francisco Isaac Fernandes Gomes, DDS, PhD student, University of São Paulo, Brazil.
On the Lookout for Answers to Solve the Pain Epidemic: We Are in This Together!
"Please, you need to give my mom some money back as she doesn’t have any,“ said the little girl politely to the toy store owner while her mother was paying for a birthday present. The owner looked surprised and with great empathy at the girl, and told the little girl that she could pick one toy from the shelf for herself. The girl kept the present for years, which always reminded her of the kind-heartedness that woman showed her family in a time of need. That girl was me.
Unexpectedly, 29 years later, I walk the corridors of the University of Calgary at Foothills Campus, though only once a week because of the current COVID-19 situation. Everything is quieter than it used to be, but that gives me time for reflection. The other days I find myself behind my Lenovo laptop in my home office looking out at the skyline of downtown Calgary, thinking about how lucky I am to be here or pondering over a problem I am trying to solve. I am most often seeking to solve diagnostic problems by using bioinformatics approaches. I search for answers to the big questions by analyzing large-scale biological data, particularly focusing on -omics and imaging. When I am not at work trying to improve diagnoses for Indigenous families with undiagnosed genetic conditions, in my spare time I am working on questions such as, How can we more accurately diagnose patients with headaches and select more optimal treatment strategies?
I researched primary headaches during my PhD studies, carried out on Danish and Swedish lands. Not only did I learn about the academic world and its unwritten rules, but I also climbed the enormous mountain of knowledge created by researchers over decades. I found that when you are looking for answers, you often end up having more questions than when you started the journey. I was on the lookout for answers on how to make individualized treatment plans, and I still am, as my mom has migraine and cluster headache (both are primary headaches), and has had difficulties getting the help she needs. I believe other headache patients are in the same boat. Being diagnosed with deep infiltrating endometriosis, I also know a thing or two about living with pain. Of course, pain does not only affect me and my family; there are likely more than a billion people in pain worldwide (about 1 in 5 people).
I want to help find and share potential pharmacological and non-drug solutions via my research and science communication. I cannot think of anything I would rather do than help solve the pain epidemic. I always ask myself, How can I do better tomorrow? with regard to personal leadership, asking better questions, and improving my research practices. By doing that, I will continue to progress every day, which hopefully will allow me to help others live a better and happier life.
Simona Denise Frederiksen, postdoctoral associate, University of Calgary, Canada.
Harnessing the Power of Human Connection to Manage Pain
Hello, PRF readers! My name is Manasi Mittinty. I am a physician-scientist at the Pain Management Research Institute, University of Sydney.
I was introduced to pain at the age of 15 years through a fire accident that caused me third-degree burns. I vividly remember that of all the symptoms I experienced, pain was the most distressing. I would often ask my doctors, “When will my pain go away?” They would assure me that as my wounds healed my pain would subside and then disappear! I waited for days, which turned into months, and then years! Pain was and is still a companion!
This experience put me on a quest, through my medical training and then later in practice and research, to gain a deeper understanding of pain. I am particularly interested in understanding what makes the experience of pain so subjective. We know for sure that the pain experience is not limited to our innate physiology, but also requires a broader biopsychosocial perspective to understand the individuality of the experience of pain.
Thinking of my accident from all those years back still creates a feeling of triumph and courage in me; I have never looked at it as a barrier. Thanks to my loving and supportive family, it was never “me” coping with the injuries but “us” learning to accommodate the changes the accident had brought on. That may be the key reason why my pain is my companion: It is a reminder of strength, not struggle.
For this very reason my research focuses on exploring how relationships affect coping and thereby recovery. Can building and strengthening meaningful relationships be a viable solution to improve patients’ quality of life? This is a particularly important issue since many people with chronic pain become very isolated, which makes it harder to cope.
People all over the world are surviving the pandemic by finding different ways to hold onto togetherness. Meaningful connectedness and attachment amazingly anchor growth and healing in the most traumatic of situations.
It is this innate power of relationships that I desire to harness through my research, focusing on creating programs that educate people about how relationships can both support and detract from recovery in the context of pain. My vision is to see patients and their families not only cope well with pain, but also thrive and enjoy living despite being in pain.
I am thrilled to be part of the PRF Virtual Correspondents Program and have a platform to connect with the wider PRF family over the coming months. Follow me on Twitter @DrManasiMurthy.
Manasi M Mittinty, lecturer, University of Sydney, Australia.
A Focus on Spinal Cord Injury Pain
Hello, PRF readers! My name is Morgan Sharp, and I am a (soon-to-defend) PhD student in the translational neuroscience program at the University of Louisville. After completing my undergraduate degree in biomedical engineering, I began my doctorate in 2018 as part of the Kentucky Spinal Cord Injury Research Center (KSCIRC) under the mentorship of Dr. David Magnuson. Our laboratory is well known for conducting translational research regarding the efficacy of various clinical interventions after spinal cord injury (SCI) on functional recovery. While this research primarily encompasses locomotor recovery, I have particularly become interested in the topic of neuropathic pain and plasticity after SCI, and have thus found a passion I plan to pursue as an independent investigator one day.
I currently investigate peripheral anatomical plasticity of afferents after spinal cord injury. Following SCI, it is well established that primary afferents, especially nociceptors, undergo widespread, multi-segmental sprouting within the spinal cord. However, very little is known about nociceptor plasticity within peripheral muscles and fascia after SCI, a pathology seemingly conducive for sprouting. Using a novel virus that we have developed to label peripheral afferents, I plan to investigate changes in the morphology of primary afferents innervating muscles and fascia after SCI, and determine whether peripheral sprouting is a mechanism underlying sensitization and neuropathic pain.
KSCIRC has provided me with a unique research experience, as we are in close proximity to the well-known Frazier Rehabilitation Institute. As a result, I have had numerous opportunities to learn from physicians and clinical researchers, interact with patients, participate in rehabilitation sessions, and ultimately design translational experiments. Our lab has worked with physical therapists from the Frazier Rehabilitation Institute to implement physical therapy-based muscle stretching for rodents (yes, you read that correctly) following SCI and have found that muscle stretching is actually detrimental to functional recovery. As part of my dissertation, I plan to investigate whether muscle stretching after SCI exacerbates central and peripheral nociceptor sprouting, how this contributes to the stretching phenomenon, and the efficacy of pharmacological strategies in augmenting this plasticity.
Through my experiences in interacting with diverse groups of people and conducting translational research, I have realized how crucial effective scientific communication is, especially in the field of pain, where patients are seeking relief and support. Furthermore, I believe that COVID-19 has exposed the importance of accurately disseminating scientific findings to the public. Therefore, I am extremely humbled to have been selected as a Correspondent for the Pain Research Forum. The past year has been a valuable lesson in the absolute necessity for science to be communicated as effectively to the public as it is among researchers, and learning this skill in the field I am passionate about will be paramount for my future career as a scientist. I hope to provide thought-provoking topics that we can use to learn from each other, generate productive conversations, and become a closer community – all in the name of pain!
Stay tuned for more!
Morgan Sharp, PhD Student, University of Louisville, US.


