Introduction
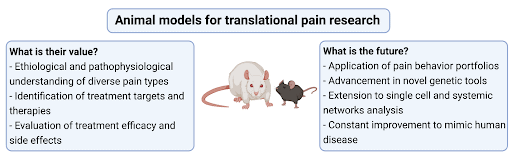
Animals are used in preclinical research to study the etiology and pathophysiology of pain, unravel signal transduction pathways, identify, and evaluate new target molecules and regions, develop therapeutic strategies, and analyse their efficacy. Animal experimentation offers the advantage that factors relevant to acute pain and factors involved in the development and maintenance of chronic pain can be systematically investigated to find causal relationships, which is often not possible in humans. Examples of animal experimentation include the investigation of genetic, molecular, and cellular mechanisms of pathological pain conditions [5]. Researchers are also developing and using preclinical animal models to assess the sensory and psychological complexities of chronic pain [1; 7](Figure 1).
Animal models of injury and diseases have been developed to investigate pain processes and potential treatments.
Pain is a multi-dimensional experience which can differ widely from patient to patient based upon the disease or type of injury, and the part of the body affected. Several animal models mimicking many acute and chronic pain conditions have been developed. Different species, sexes, and ages of animals are used, and many other important translational factors are considered in the design of studies. Rodents are mostly used because they show a high degree of nervous system and genetic similarities to humans e.g., Zheng-Bradley et al. [11]. A broad spectrum of models is available, and these are constantly expanded and modified in response to clinical need [1; 4; 7]. The models have enabled investigation of the mechanisms underlying neuropathic pain, the role of inflammation in painful conditions, such as arthritis, and even enabled investigation of complex syndromes, like fibromyalgia and lower back pain. Behavioral assessments are used to evaluate experimental outcomes. They are categorized into reflexive, non-reflexive, free-choice tests, or non-evoked voluntary behaviors, and relate to assessing the varied sensory and emotional experiences of pain [8; 9]. Reflexive assessments measure sensitivity to a mechanical, thermal, or cold stimuli. Non-reflexive assessments assess physical function like locomotor activity which may correlate with pain. Free-choice assessments are used to correlate pain with reward processes. Investigation of home cage behavior or the performance of voluntary motivational tasks like wheel running can give insights into the well-being of animals.
How closely an animal model resembles facets of the human condition is important.
There are several ways in which animal models differ from patients’ clinical experience, which limits how well findings from animal experiments can be translated. Firstly, pain is often studied in young, healthy, genetically similar male animals. This contrasts with the clinical situation in which pain predominantly occurs in middle-aged or elderly female patients with comorbidities, polypharmacy and with heterogenous genetic background. Secondly, animals do not effectively simulate the multidimensional nature of clinical pain conditions, which are affected by complex psychological components, social parameters, education level, and environmental factors. Thirdly, animal models lack the degenerative nature of human chronic diseases that progress over years rather than weeks, as is the case in most laboratory experiments. Finally, pain cannot be directly measured in animal models; researchers have to rely on surrogate behaviors, whereas patient pain is measured through self-reporting [10].
Animal experimentation must be conducted in accordance with ethical guidelines.
During the decision making of whether an experiment is justified by an independent institutional review board, the likely pain and distress experienced by animals will be minimized and weighed against the potential scientific benefits. The 3Rs framework (reduction, refinement and replacement) is used to assess and embed minimizing harm to animals used in research [6]. Replacement refers to technologies that replace or avoid the use of animals in experiments. Reduction refers to methods that minimize the number of animals used in an experiment without undermining the scientific aims. Refinement refers to methods that minimize suffering. It applies to all aspects of animal use, from their housing to the scientific procedures.
Animal research has made translation and recent drug developments for some pain conditions possible.
Several new drugs have been introduced in recent years based on evidence from animal research. For example, calcitonin gene related peptide (CGRP) was discovered in 1982 [2]. In subsequent animal studies, its role in the trigeminovascular reflex as the basis of migraine was characterized. In parallel human studies demonstrated the release of CGRP into the jugular venous plexus during a migraine attack. Since 2018, CGRP antibodies and antagonists have been approved for the prevention of migraine. A further example are isoenzyme-specific cyclooxygenase-2 (COX-2) inhibitors to treat pain associated with high levels of inflammation[3]. Animal models helped to identify Cox-2 in brain tissue and its upregulation in response to inflammation in the 1990s. As a result, celecoxib (a COX-2 inhibitor) is widely approved as an inflammatory pain treatment.
There have been some drugs that showed promising results in animal studies but have failed in the clinic due to species differences. This is addressed in the fact sheet “Human cells and tissue in preclinical studies: the DRG”
Researchers are continuously working to improve the accuracy and reliability of animal models.
The challenge for preclinical pain researchers is to sufficiently model in animals’ aspects of the complexity of the patient pain experience to investigate and improve our understanding of the underlying mechanisms. Several technological advances are being employed to improve our understanding of pain processes, e.g., single-cell investigations and high-resolution in vivo imaging. Scientists continue to develop and refine animal models to simulate the underlying disease and clinical presentation of pain more closely. Potential treatments are assessed using a wider range of behavioural assessments to include sensory and psycho-social changes due to pain. Additionally, systematic review and meta-analysis of animal studies help to obtain a comprehensive view of the validity and utility of studies. The future aim is to close the translation gap, so potential therapies effective in animal experiments are also effective for patients.
REFERENCES
[1] Burma NE, Leduc-Pessah H, Fan CY, Trang T. Animal models of chronic pain: Advances and challenges for clinical translation. Journal of Neuroscience Research 2017;95(6):1242-1256.
[2] Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies – successful translation from bench to clinic. Nat Rev Neurol 2018;14(6):338-350.
[3] Flower RJ. The development of COX2 inhibitors. Nat Rev Drug Discov 2003;2(3):179-191.
[4] Henze DA, Urban MO. Large Animal Models for Pain Therapeutic Development. In: L Kruger, AR Light, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis
Copyright © 2010 by Taylor and Francis Group, LLC., 2010.
[5] Lacroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annual review of pharmacology and toxicology 2009;49:97-121.
[6] NC3Rs, BBSRC, Defra, MRC, NERC, Society R, Trust W. Responsibility in the use of animals in bioscience research: expectations of the major research councils and charitable funding bodies. 2019.
[7] Sadler KE, Mogil JS, Stucky CL. Innovations and advances in modelling and measuring pain in animals. Nature reviews Neuroscience 2021.
[8] Tappe-Theodor A, King T, Morgan MM. Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci Biobehav Rev 2019;100:335-343.
[9] Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents–challenges and opportunities. Eur J Neurosci 2014;39(11):1881-1890.
[10] Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain 2008;135(1-2):7-10.
[11] Zheng-Bradley X, Rung J, Parkinson H, Brazma A. Large scale comparison of global gene expression patterns in human and mouse. Genome Biology 2010;11(12):R124.



