Our lab continues to be intrigued by the mechanisms that generate and drive skeletal pain. Painful skeletal conditions are highly prevalent and their impact is pervasive in both developing and developed countries (Lubeck, 2003;Woolf & Pfleger, 2003; Brooks, 2006; Kidd, 2006). The skeletal system is essential for structural support, movement, protection of internal organs, mineral and growth factor storage and release, and the birth and maturation of blood and immune cells. Skeletal pain occurs in a diverse group of disorders including osteoporosis, vertebral degeneration, osteoarthritis, fibrous dysplasia, and Sickle cell disease. Importantly, if skeletal pain is not adequately controlled it often has secondary effects including loss of bone and muscle mass, cardiovascular function and cognitive health, all of which can significantly diminish the patient’s functional status and quality of life.
Skeletal pain tends to increase with age as the mass, quality and strength of the human skeleton peaks at 25–30 years of age in both males and females (Heaney et al., 2000; Seeman, 2002) and then declines thereafter. With increasing lifespans in developing and developed countries, and with the rise of sedentary lifestyles both of which reduce skeletal health, the burden that skeletal pain will exact on individuals and society is expected to increase markedly in the coming decades (Peltonen et al., 2003; Stovitz et al., 2008).
One major reason for why chronic skeletal pain is debilitating is that we currently have very few therapies that can attenuate this pain without significant, unwanted side-effects. While both nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates have been shown to be effective in attenuating acute skeletal pain, long-term use of these agents for managing chronic nonmalignant skeletal pain is problematic (Martell et al, 2007; Crofford, 2010; Sullivan and Howe, 2013; Helmerhorst et al, 2014).
We have recently focused on the important question as to whether there is a different peripheral mechanism driving acute vs. chronic pain (Chartier et al., 2014).
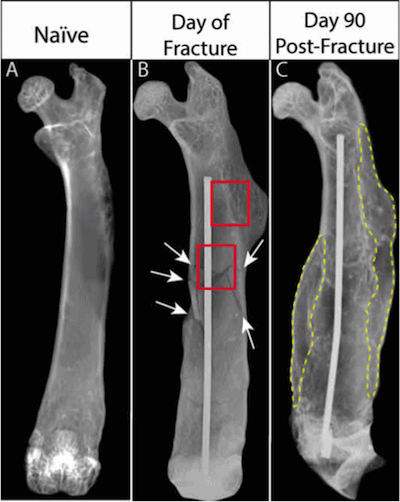
Radiographic images of the same femur at 3 time points: naïve (before fracture), immediately post fracture, and 90 days post-fracture. White arrows indicate fracture lines. Red outlined boxes indicate where bright field and confocal images for Figs. 2 and 3 were acquired. Note that in (B) there is an extensive series of fracture lines and in (C), the same bone 90 days after fracture, complete union of the mineralized bone has yet to occur and the partly mineralized, cartilaginous callus (yellow outline) has yet to be fully resorbed.
Using a mouse model we observed that following a bone fracture which does not fully heal (Figure 1), there is pathologic sprouting and increased density of both sensory and sympathetic nerve fibers in the nonhealed fracture and formation of neuroma-like structures near the fracture site (Figure 2).
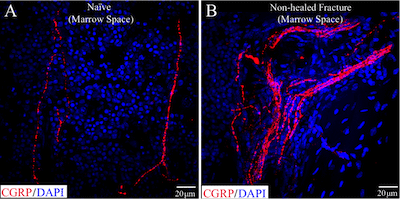
Sprouting of CGRP+ sensory nerve fibers and the formation of neuroma-like structures in the bone marrow of a nonhealed, fractured femur. Note the increased heterogeneity and density of CGRP+ nerve fibers (red) in bone marrow of fractured femurs (B) compared to naïve femurs (A).
Importantly, all of the animals with nonhealed fractures that had ectopic sprouting displayed significant pain behaviors upon palpation of the nonhealed fracture. In contrast, similar aberrant nerve growth or palpation-induced pain behaviors were never observed in normal femurs. Interestingly, we observed that these neuroma-like structures were typically observed in close proximity to mineralized bone “pockets” in the normally homogenous marrow space of nonhealed, fractured femurs (Figure 3). This novel finding suggests that the disruption of the normally homogenous microenvironment of the bone can create a permissive environment allowing for the sprouting of sensory and sympathetic nerve fibers, and ultimately, the generation and maintenance of chronic skeletal pain.
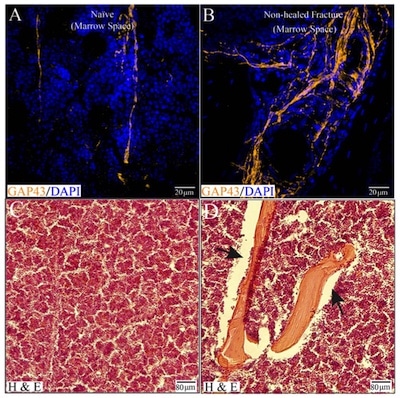
Sprouting of nerve fibers and ectopic mineralized bone formation and in the marrow space of nonhealed, fractured femurs. In the normal bone, the microenvironment of bone is homogenous, with marrow, mineralized bone, and periosteum each occupying their distinct compartment. Following a traumatic fracture, as modeled in the study, this normally homogenous environment becomes disrupted. The homogenous bone marrow is typically comprised of hematopoietic tissue, adipose cells, and lymphoid tissue (C). Note that in the marrow space of the nonhealed fracture, mineralized bone (see black arrows) has infiltrated the normally homogenous bone marrow and is closely localized with sprouted Gap43+ nerve fibers (organge) (B, D).
Currently there is a lack of research which addresses the specific cellular factors that maintain a nonpermissive or permissive environment for this presence and sprouting of sensory and sympathetic nerve fibers in the skeleton. However a few potential mediators are emerging.
Given the novelty of our current findings, several additional questions arise: What is the functional significance of sensory and sympathetic nerve sprouting? Does nerve sprouting contribute to the transition from acute to chronic skeletal pain? How to age and gender factor into the development and maintenance of chronic skeletal pain? Does the nerve sprouting return to “normal” conditions if a fracture is healed? Our ongoing research seeks to address these important questions.
About Stephane Chartier
 Stephane Chartier is a post baccalaureate research assistant and imaging specialist in the Department of Pharmacology at the University of Arizona. Using the latest confocal techniques, he works at the interface of neurobiology, bone biology, and stem cell biology to help address the growing clinical problem of chronic musculoskeletal pain. More recently, his research efforts have focused on clarifying the mechanisms that contribute to chronic skeletal pain after tissue injury in the young and aging individual. He aspires to enter the medical field and continue addressing the needs of those affected by this debilitating pain.
Stephane Chartier is a post baccalaureate research assistant and imaging specialist in the Department of Pharmacology at the University of Arizona. Using the latest confocal techniques, he works at the interface of neurobiology, bone biology, and stem cell biology to help address the growing clinical problem of chronic musculoskeletal pain. More recently, his research efforts have focused on clarifying the mechanisms that contribute to chronic skeletal pain after tissue injury in the young and aging individual. He aspires to enter the medical field and continue addressing the needs of those affected by this debilitating pain.
References
- Brooks PM. The burden of musculoskeletal disease–a global perspective. Clinical rheumatology. 2006;25(6):778-81. doi: 10.1007/s10067-006-0240-3. PubMed PMID: 16609823.
- Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain. 2014;155(11):2323-36. doi: 10.1016/j.pain.2014.08.026. PubMed PMID: 25196264; PubMed Central PMCID: PMC4254205.
- Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nature reviews Rheumatology. 2010;6(4):191-7. doi: 10.1038/nrrheum.2010.24. PubMed PMID: 20357788.
- Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985-1009. doi: 10.1007/s001980070020. PubMed PMID: 11256898.
- Helmerhorst GT, Vranceanu AM, Vrahas M, Smith M, Ring D. Risk factors for continued opioid use one to two months after surgery for musculoskeletal trauma. J Bone Joint Surg Am. 2014;96(6):495-9. doi: 10.2106/JBJS.L.01406. PubMed PMID: 24647506.
- Kidd BL. Osteoarthritis and joint pain. Pain. 2006;123(1-2):6-9. doi: 10.1016/j.pain.2006.04.009. PubMed PMID: 16714085.
- Lubeck D. The costs of musculoskeletal disease: health needs assessment and health economics. Best Practice & Research Clinical Rheumatology. 2003;17(3):529-39. doi: 10.1016/s1521-6942(03)00023-8.
- Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Annals of internal medicine. 2007;146(2):116-27. PubMed PMID: 17227935.
- Peltonen M, Lindroos AK, Torgerson JS. Musculoskeletal pain in the obese: a comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain. 2003;104(3):549-57. PubMed PMID: 12927627.
- Seeman E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology. 2008;47 Suppl 4:iv2-8. doi: 10.1093/rheumatology/ken177. PubMed PMID: 18556646; PubMed Central PMCID: PMC2427165.
- Stovitz SD, Pardee PE, Vazquez G, Duval S, Schwimmer JB. Musculoskeletal pain in obese children and adolescents. Acta paediatrica. 2008;97(4):489-93. doi: 10.1111/j.1651-2227.2008.00724.x. PubMed PMID: 18363957.
- Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154 Suppl 1:S94-100. doi: 10.1016/j.pain.2013.09.009. PubMed PMID: 24036286.
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization. 2003;81(9):646-56. PubMed PMID: 14710506; PubMed Central PMCID: PMC2572542.



