Macrophages are found in all tissues and are critical to injury and repair. Like neurons, macrophages are plastic and acquire different phenotypes based on the external environment. There are two types of macrophages, M1 which secrete inflammatory cytokines, and M2 which secrete anti-inflammatory cytokines. Inflammatory cytokines activate nociceptors and promote pain and anti-inflammatory cytokines inhibit nociceptors and promote analgesia. Two recent publications by our group showed the critical role that macrophages may play in both the production of chronic musculoskeletal pain and in the analgesia produced by regular physical activity [1,2].
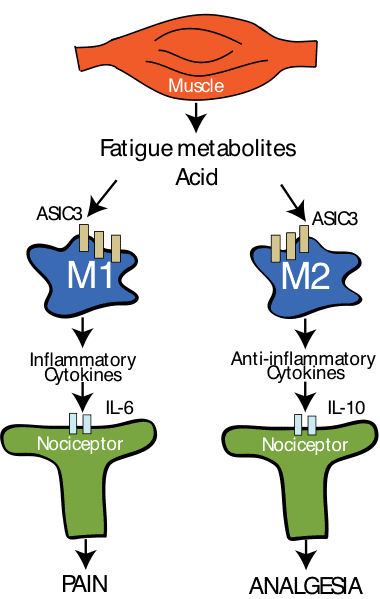
Figure 1, shows our theory that suggests macrophage phenotype dictates whether pain or analgesia predominates after injections of acidic pH. In otherwise sedentary animals there is a greater proportion of M1 macrophages while in physically active animals there is an increase in M2 macrophages.
We have developed a model of chronic widespread musculoskeletal pain induced by giving 2 injections of pH 4 saline into the gastrocnemius muscle. Behavioral responses to the muscle insultare tested by examining withdrawal thresholds of the paw or muscle as a measure of hyperalgesia, and measuring physical activity levels using an open field test. This model results in muscle and paw hyperalgesia that spread to the contralateral limb, enhanced visceral sensitivity, and reduced activity. As in people with chronic musculoskeletal pain there is no tissue damage or inflammation. The hyperalgesia is sensitive to pharmaceutical and non-pharmaceutical therapies with a similar pattern to that observed in human subjects with chronic widespread pain – opioids, reuptake inhibitors, gabapentinoids, and exercise are effective; NSAIDs and channel blockers are ineffective.
Using this model we asked if macrophages were involved in the development of hyperalgesia in our chronic muscle pain model. We showed that the number of macrophages in the muscle increased after induction of the model and that when we removed the macrophages in muscle, using clodronate liposomes, the development of hyperalgesia was prevented –suggesting a role for macrophages in development of chronic pain. We then asked if activation of macrophages would mimic the effects of repeated saline injection. To test this we replaced one of the pH 4 injections with a macrophage activator, lipopolysaccharide (LPS), and tested pain responses. LPS, which activates Toll-like receptors (TLR – receptors that have a key role in innate immunity) found on macrophages, in combination with one pH 4 injection reproduced the hyperalgesia produced by repeated acid injections. In contrast, blockade of macrophage activity, with a TLR antagonist, prevented the development of hyperalgesia. Together these data provide strong evidence of the role of macrophages in development of hyperalgesia.
Next we asked if the inflammatory cytokine, IL-6, reproduced the hyperalgesia and induced the release of inflammatory cytokines. We therefore, replaced one injection of pH 4 with IL-6 and tested pain responses. IL-6 combined with one pH 4.0 injection reproduced the hyperalgesia produced by repeated acid injections. We wanted to investigate whether pH 4 induced the release of inflammatory cytokines. To do this, we cultured macrophages, treated them with acidic saline or LPS, and measured the inflammatory cytokine release (using multiplex analysis). Treatment with acidic saline increased the release of inflammatory cytokines, including IL-6, similar to that produced by activating macrophages with LPS.
Importantly all of these experiments were done in sedentary animals. In fact, nearly all basic science studies are done in sedentary animals, and this is itself a risk factor for the development of chronic musculoskeletal pain in humans[3]. On the other hand, regular physical activity, reduces the risk for development of chronic musculoskeletal pain in humans[3,4]. Further, it is well known that exercise is an effective treatment for chronic muscle pain conditions, like fibromyalgia. We therefore asked whether regular physical activity prevented the development of chronic musculoskeletal pain. To do this, we placed running wheels in the home cages of mice and compared them to mice housed in their normal cages. After 8 weeks of regular physical activity, 2 injections of acidic saline did not produce hyperalgesia when compared to sedentary mice[1,5].
We hypothesized that regular physical activity released factors from the exercising muscle that would alter the phenotype of muscle macrophages. To test this we examined the number of macrophages that expressed either an M1 marker or an M2 marker, using immunohistochemistry. In physically active animals there was an increase in the number of macrophages that expressed the M2 markers, suggesting a change in phenotype. Since M2 markers release anti-inflammatory cytokines, we proposed that IL-10, an anti-inflammatory cytokine, mediated the analgesia produced by regular physical activity. To test this we gave an antibody to the IL-10 receptor either systemically or directly into the muscle in physically active animals with the chronic muscle pain model. Systemic blockade of IL-10 prevented the bilateral analgesia in physically active mice. Unilateral blockade of IL-10 – into the muscle that received the pH 4 injections – also prevented the analgesia. Interestingly, if the blocker was given to the contralateral muscle, the analgesia in that muscle was also prevented.
Lastly, we asked if IL-10 in sedentary animals would also prevent the development of hyperalgesia. IL-10 delivered either systemically, or directly to the muscle for 10 days before induction of the model, prevented the development of hyperalgesia. These data together suggest that the effects of regular physical activity are to increase M2 macrophages in muscle tissue, which results in an increase in IL-10 in muscle that reduces nociceptor activity.

In summary, we propose that the state of the immune system influences the development of chronic muscle pain. The immune system is a balance between inflammatory and anti-inflammatory processes and the net output of these two processes determines the response to muscle insult (Figure 2). Under sedentary conditions, the system is set up to enhance the release of pro-inflammatory cytokines and produce pain to mild muscle insults. On the other hand, with regular physical activity, the immune system is in an anti-inflammatory state, and prevents the development of hyperalgesia. If this mechanism applies in humans and is an important driver of chronic clinical pain states, then as clinicians we could capitalize on this effect by recognizing the underlying mechanisms and promoting activity to facilitate an anti-inflammatory state. Understanding the mechanisms can also be used to help educate the patient on the importance of exercise and provide a rationale for them to participate in a regular exercise program.
About Kathleen A. Sluka
 Dr Sluka is a professor in the Department of Physical Therapy and Rehabilitation Science at the University of Iowa. She received a physical therapy degree from Georgia State University and a PhD in Anatomy from the University of Texas Medical Branch in Galveston. After a postdoctoral fellowship with Dr. William D. Willis, she joined the faculty at the University of Iowa. Dr Sluka’s research focuses on the neurobiology of musculoskeletal pain as well as the mechanisms and effectiveness of non-pharmacological pain treatments commonly used by physical therapists. She has published over 190 peer-reviewed manuscripts, numerous book chapters, and a textbook on Pain Mechanisms and Management for the Physical Therapist. She has received numerous awards including the Marian Williams Award for Research in Physical Therapy and Catherine Worthingham Fellowship from the American Physical Therapy Association and the Frederick W.L. Kerr Basic Science Research Award from the American Pain Society. She is actively involved in the International Association for the Study of Pain, the American Pain Society, and the American Physical Therapy Association serving on committees, task forces and society boards.
Dr Sluka is a professor in the Department of Physical Therapy and Rehabilitation Science at the University of Iowa. She received a physical therapy degree from Georgia State University and a PhD in Anatomy from the University of Texas Medical Branch in Galveston. After a postdoctoral fellowship with Dr. William D. Willis, she joined the faculty at the University of Iowa. Dr Sluka’s research focuses on the neurobiology of musculoskeletal pain as well as the mechanisms and effectiveness of non-pharmacological pain treatments commonly used by physical therapists. She has published over 190 peer-reviewed manuscripts, numerous book chapters, and a textbook on Pain Mechanisms and Management for the Physical Therapist. She has received numerous awards including the Marian Williams Award for Research in Physical Therapy and Catherine Worthingham Fellowship from the American Physical Therapy Association and the Frederick W.L. Kerr Basic Science Research Award from the American Pain Society. She is actively involved in the International Association for the Study of Pain, the American Pain Society, and the American Physical Therapy Association serving on committees, task forces and society boards.
Reference List
[1] Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing IL-10 in mice. Pain 2016;157:79.
[2] Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA. Resident macrophages in muscle contribute to development of hyperalgesia in a mouse model of non-inflammatory muscle pain. J Pain 2016.
[3] Landmark T, Romundstad PR, Borchgrevink PC, Kaasa S, Dale O. Longitudinal associations between exercise and pain in the general population–the HUNT pain study. PLoS One 2013;8(6):e65279.
[4] Zhang R, Chomistek AK, Dimitrakoff JD et al. Physical Activity and Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Med Sci Sports Exerc 2015;47:757-764.
[5] Sluka KA, O’Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol 2013;114(6):725-733.
Commissioning Editors: Lorimer Moseley and Carolyn Berryman



