Facilitation of central pain mechanisms is proposed to be a potential missing link between identifiable tissue damage and the severity of pain experienced across a range of painful conditions [1]. Clinically, it is purported to manifest as widespread hyperalgesia, due to impaired descending nociceptive inhibition and enhanced nociceptive facilitation [1]. At present, however, much of the literature on this topic relies on sensory assessments made at a single time point: either to show cross-sectional differences between patients with pain and pain-free individuals [2], or to predict future pain-related outcomes [3]. This means less is known about what happens to measures of sensitisation over time in the same individuals, and to what extent they are influenced by variation in other factors. Such influencing factors might include mood, sleep, anxiety, disability, disease severity or even duration [4], but what about simply the presence of pain on the day of testing? If just being in pain at the time can make someone appear sensitised, it would be no wonder that patients with pain appear different to their pain-free counterparts!
We, therefore, wanted to take pain-free individuals and test them before, during and following an experimental episode of low back pain lasting several days (induced by eccentric trunk extensions to fatigue) [5]. This would allow us to see the effect of pain presence on these measures, while also allowing us to examine if baseline measures could explain inter-individual differences in the subsequent severity of pain.
It’s probably important to mention what we were actually testing. In humans, hyperalgesia, nociceptive facilitation and inhibition can only (easily and ethically) be measured indirectly by way of psychophysical assessment. This means we assessed hyperalgesia using pressure-pain thresholds (PPTs) at local (low back) and distant (elbow, shoulder and calf) sites. Then assessed temporal summation of pain (TSP, for nociceptive facilitation) and conditioned pain modulation (CPM, for nociceptive inhibition), again using pressure, this time applied via a computer-controlled cuff algometry system. In short, TSP is assessed by applying repetitive noxious stimuli and examining increases in pain ratings across repetitions, thought to reflect increasing spinal cord excitability from repeated nociceptor activation. On the other hand, CPM is tested by comparing a painful test stimulus (e.g. pain detection threshold) applied alone, to one applied in the presence of another tonic painful conditioning stimulus somewhere else on the body (tonic cuff inflation). A normal response is to feel less pain (or detect pain later) when the conditioning stimulus is present, as a result of descending inhibitory pathways from the midbrain dampening incoming nociceptive information.
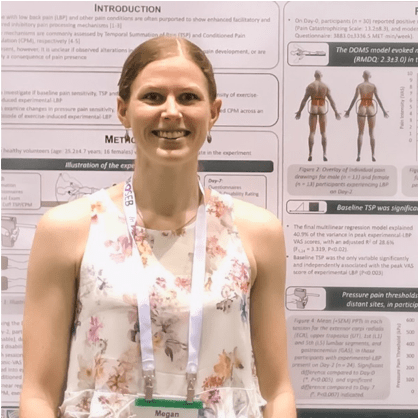
What did we find? Well, we managed to effectively produce a mild low back pain episode in all participants, which lasted long enough for our Day-2 testing session in 24 out of 30 participants. These participants with low back pain present on Day-2, showed reduced PPTs over local low back sites compared to the pain-free Day-0 and Day-7 assessments. However, they also showed reductions in PPTs at the elbow, shoulder and calf. This suggests that we induced widespread hyperalgesia, though the interpretation of this is complicated, as widespread changes could be a result of repeated testing, synergistic muscle involvement, or other factors beyond the presence of low back pain alone. Further, we did not find any significant differences between the sessions for TSP or CPM, suggesting that the presence of pain (at least with this model) was not enough to produce quantifiable sensitisation.
On the other hand, we used a multilinear regression approach to explain the inter-individual variance in subsequent peak experimental low back pain intensity (i.e. the maximum VAS score (from 0cm = no pain, to 10cm = worst pain imaginable) in the bidaily pain diary recorded during the study period). With all measured baseline variables (including pressure pain thresholds, cuff pain thresholds, TSP, CPM, number of eccentric repetitions, age, gender, mood, sleep time, physical activity, catastrophising) the only significant independent association was found for baseline TSP. This fits with prior post-operative pain prediction models [3], and to some extent with recent preliminary work in acute low back pain [6], but as always, requires more investigation.
What does this mean? Well, pain presence may influence some measures (namely pressure-pain thresholds in this study), but there is no clear effect on TSP and CPM, at least from this specific pain model. Emerging data from our lab, however, suggests that perhaps a model inducing higher pain intensity could have a stronger effect and impair CPM. So perhaps CPM specifically is more amenable to alteration in a state-dependent manner – be it due to pain presence (perhaps with higher intensities or longer duration) or variation in other factors. Alternatively, we speculate that TSP may be more stable and, based on its explanatory value in our regression model, may represent an important trait-like measure with some capacity to predict or explain differences in the severity of pain developed. Of course, we should also remember that pain induced in the lab is generally more controlled, and likely less threatening than clinical pain, so these assertions need further validation in pain populations.
Indeed, at CNAP we focus on better understanding changes in such pain mechanisms, broadly defined as pain neuroplasticity. We undertake studies which aim to provoke, probe and modulate pain neuroplasticity, with a particular focus on changes occurring in the early phase of the transition from acute to sustained pain.
About the authors
Megan McPhee
 Megan McPhee is currently a PhD fellow at the Center for Neuroplasticity and Pain (CNAP), SMI®, at Aalborg University in Denmark. She has a Bachelor of Physiotherapy (Hons) from the University of Queensland, and an MSc in Pain Management from the University of Sydney. Her research focuses on understanding the relationship between measures of nociceptive inhibition and facilitation, and the experience of low back pain over time, in both experimental models and clinical conditions. Beyond this, she is more generally interested in perceptual learning and neuroplasticity, along with the complex interplays between pain modulation, affect, attention, expectation and movement.
Megan McPhee is currently a PhD fellow at the Center for Neuroplasticity and Pain (CNAP), SMI®, at Aalborg University in Denmark. She has a Bachelor of Physiotherapy (Hons) from the University of Queensland, and an MSc in Pain Management from the University of Sydney. Her research focuses on understanding the relationship between measures of nociceptive inhibition and facilitation, and the experience of low back pain over time, in both experimental models and clinical conditions. Beyond this, she is more generally interested in perceptual learning and neuroplasticity, along with the complex interplays between pain modulation, affect, attention, expectation and movement.
Prof. Thomas Graven Nielsen
Thomas Graven Nielsen
Thomas Graven-Nielsen received an M.Sc.EE degree within Biomedical Engineering from Aalborg University, Denmark in 1994, and acquired his PhD within Biomedical Science and Engineering in 1997. In 2006, he obtained a Doctoral degree in Medical Science from Copenhagen University. He is now Director at Center for Neuroplasticity and Pain (CNAP, funded by the Danish National Research Foundation), Department of Health Science and Technology, Aalborg University, Denmark (since 2015), and has been a Full Professor in Pain Neuroscience since 2008. His research focuses on translational studies of musculoskeletal pain, bridging the gap between basic animal findings and clinical manifestations of pain. The scope is to identify and modulate key features of human pain neuroplasticity to prevent maladaptive neuroplasticity and promote advantageous neuroplasticity. Development of pain models, bio-markers, and assessment technologies are key biomedical tools for the translational studies. The core areas are muscle pain, joint pain, referred pain, localised and widespread deep-tissue hyperalgesia, pharmacological screening, and electrophysiological techniques to assess muscle pain physiology and neuroplasticity. He has published 325+ papers and reviews (275+ peer-reviewed, H-factor: 55) and received several awards.
References
[1] Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Mohr Drewes A. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain. 2018 Feb;22(2):216-241. DOI: 10.1002/ejp.1140. Epub 2017 Nov 5.
[2] O’Brien AT, Deitos A, Triñanes Pego, Fregni F, Teresa Carrillo-de-la-Peña M. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. JPain. 2018 Aug;19(8):819-836. DOI: 10.1016/j.jpain.2018.01.010
[3] O’Leary H, Smart KM, Moloney NA, Doody CM. (2017), Nervous System Sensitization as a Predictor of Outcome in the Treatment of Peripheral Musculoskeletal Conditions: A Systematic Review. Pain Pract. 2017 Feb;17(2):249-266. DOI: 1111/papr.12484
[4] Hermans L, Van Oosterwijck J, Goubert D, Goudman L, Crombez G, Calders P, Meeus M. Inventory of Personal Factors Influencing Conditioned Pain Modulation in Healthy People: A Systematic Literature Review. Pain Pract. 2016 Jul;16(6):758-69. DOI: 10.1111/papr.12305
[5] McPhee M, Graven-Nielsen T. Alterations in Temporal Summation of Pain and Conditioned Pain Modulation across an Episode of Experimental Exercise-induced Low Back Pain. JPain. 2018 Article in press. DOI: 10.1016/j.jpain.2018.08.010
[6] Marcuzzi A, Wrigley PJ, Dean CM, Graham PL, Hush JM. From acute to persistent low back pain: a longitudinal investigation of somatosensory changes using quantitative sensory testing—an exploratory study. Pain Reports. 2018;3(2):e641. DOI: 10.1097/PR9.0000000000000641.