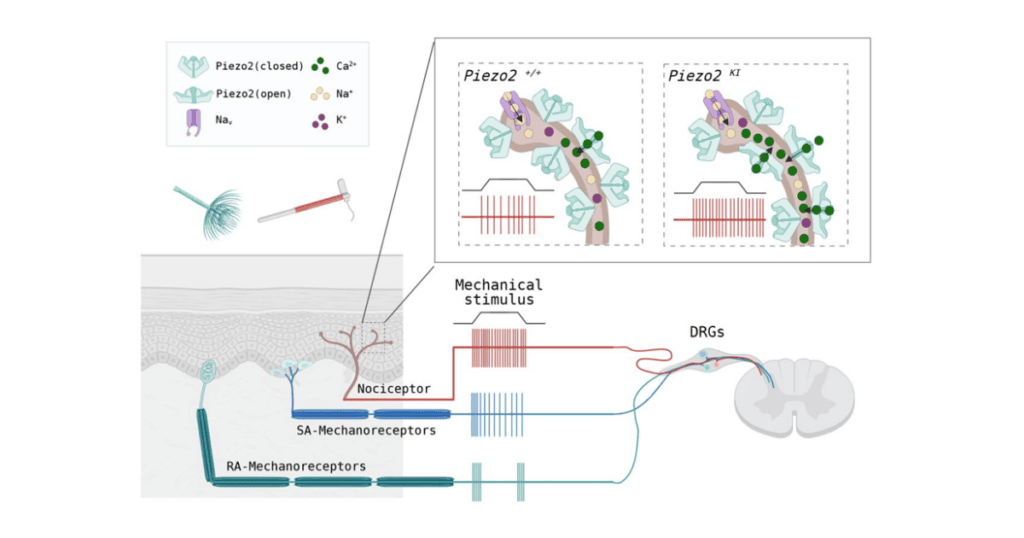
A new study reveals that removing the voltage-gated block on PIEZO2 channels leads to elevated and long-lasting nociceptor activity in response to mechanical stimulation.
The Piezo2 protein, a mechanically gated ion channel, has a well-established role in the sense of touch in both mice and humans. Previously, research had shown that loss-of-function mutations in the Piezo2 gene result in hyposensitivity to non-painful touch or vibrational stimuli, whereas gain-of-function mutations frequently resulted in complex developmental disorders. Whether these gain-of-function mutations also contribute to mechanical hypersensitivity had not yet been established.
Thanks to a paper on a study led by Oscar Sánchez-Carranza – a postdoctoral researcher from Gary Lewin’s laboratory (Max Delbrück Center for Molecular Medicine, Berlin, Germany) – that has now changed. Using two mouse strains with different gain-of-function mutations in the Piezo2 gene, the research team identified that the mutations made the nociceptors significantly more sensitive to mechanical stimuli – which may contribute to chronic pain hypersensitivity.
These results – published in Brain on 10 July 2024 – represent an important step forward in understanding the role of Piezo2 in mechanosensory touch and pain, according to Tibor Rohacs (Rutgers New Jersey Medical School, USA). “It’s not as trivial as you would think it is, because the problem with mice is that they don’t talk, so we can’t ask them whether it hurts or not,” said Rohacs, an expert on mechanically activated ion channels who was not involved in the current research.
“The same [behavioral] assay is sometimes interpreted as pain [and] sometimes interpreted as touch. You’ve probably experienced this yourself if you strain your ankle – the same movement that’s completely normal on your uninjured side is incredibly painful on the other side. So there’s a kind of nuance to it that I don’t think we’ve fully elucidated yet.”
Narrowing the list of potential candidate mutations
Lewin’s lab had previously reported that PIEZO2 channels could only be fully opened by mechanical forces with a highly positive membrane voltage, and many of these channels would remain closed while there is a negative (resting) membrane potential. The next question in this line of research was to figure out what physiological function, if any, was associated with this biophysical process.
Sánchez-Carranza created three genetically modified cell lines, each with a different single gain-of-function mutation: A highly conserved arginine residue in their Piezo2 gene was replaced with either histidine (R2756H), cystine (R2756C), or lysine (R2756K). These mutations are most commonly associated with developmental disorders in humans (e.g., distal arthrogryposis, Gordon syndrome, etc.).
During electrophysiology experiments, it was found that cells expressing the mutant PIEZO2 channels displayed significantly fewer rapidly adapting mechanically gated currents, along with greater intermediate and slowly adapting currents compared to wild-type cells.
Interestingly, only the R2756H and R2756K mutant channels displayed significantly slower channel inactivation and deactivation, increased mechanosensitivity, and a near total removal of the voltage block compared to the wild-type. Sánchez-Carranza et al. predicted these mutations would have similar effects on mechanosensitivity if they were introduced to an in vivo mouse model.
Similar in some ways, different in others
After generating two mouse lines that globally expressed the R2756H and R2756K mutations in the PIEZO2 channels, researchers noted an abnormal spine curvature resembling scoliosis – a trait associated with the R2756H mutation in humans – in roughly half of the homozygous R2756K mice, but not the R2756H mice. No scoliosis was observed in any of the heterozygous mice, and similarly, no joint abnormalities similar to distal arthrogryposis were seen in any of their strains.
Rohacs was surprised by these findings, noting that the human version of disease is autosomal dominant – meaning only one copy of the mutated gene is required to cause symptoms.
Current clamp recordings from cultured wild-type and mutant mechanoreceptors revealed the point mutations had not affected sensory neuron excitability, as measured through resting membrane potentials as well as rheobase and input resistance.
However, differences began to arise when researchers explored the role of the mutated PIEZO2 channels in pain sensitivity. For example, the mutated PIEZO2 channels had substantially lower current thresholds required for activation of nociceptive neurons and had an increase in the frequency at which a mechanical stimulus evoked currents from the channel. Together, these findings suggest that the mice with Piezo2 mutations display significant mechanical hypersensitivity.
Examining intact Aδ- and C-fiber nociceptors innervating the glabrous skin of mice revealed that both types of mechanonociceptors displayed significantly lower activation thresholds and significantly higher firing rates in response to a stimulus. This increased firing persisted longer in mutated C-fiber nociceptors after the stimulus was removed compared to wild-type C-fibers.
“You pretty much need to crush the skin to activate nociceptors,” Sánchez-Carranza told ScienceDaily. “But the nociceptors from the transgenic mice were triggered by levels of mechanical force that would normally be perceived as a touch. They were incredibly sensitive.”
“By changing one amino acid in Piezo2, we can actually mimic a lot of what happens in chronic pain in the C-fibers,” said Lewin, suggesting that Piezo2 could potentially be implicated in other chronic pain syndromes where C-fiber nociceptors are hyperactive, such as fibromyalgia.
The knock-in mice also displayed enhanced pain responses to mechanical stimuli. When tested using standardized behavioral assays and using paw withdrawal thresholds in response to punctate stimulation, the level of stimulation required to elicit a response was about half that of the wild-type mice.
“Mechanical sensitivity was affected to a much higher extent in nociceptive DRG [dorsal root ganglion] neurons than in mechanoreceptors,” Rohacs commented. “Accordingly, the mice showed no difference in a light touch assay (brushing the hindpaw) but showed a reduction in the von Frey filament assay.”
Importantly, the mutated mice did not display differences in beam and ladder walking tests or naturalistic digging, nor were there differences in withdrawal latencies to noxious heat stimuli – confirming the specific effects of the Piezo2 mutation on mechanical pain sensitivity.
A potential new target for analgesic development?
The authors concluded with a suggestion that their findings make the PIEZO2 channel a potential target for novel pain therapies, highlighting that a substantial amount of time, effort, and money had been invested into developing products designed to target voltage-gated sodium channels with limited success.
“By addressing the root cause of nociceptor sensitization, new drugs could provide better relief for chronic pain sufferers,” said Lewin.
Rohacs agreed, as no selective inhibitors of PIEZO2 currently exist, which would not inhibit PIEZO1, a close relative of PIEZO2, and noted there was a long way to go on this front. “PIEZO1 and PIEZO2 are similar, and it’s kind of hard to make a selective inhibitor against PIEZO2. Given that PIEZO1 is so widely expressed, a non-selective inhibitor will likely create problems in other organs,” he told Pain Research Forum.
While Rohacs felt the study had no major limitations, he did feel that the mild recapitulation of the human skeletal phenotype in mice raises the question of whether the mouse data truly reflect biological processes in humans, suggesting this would be a valuable area for future research to focus on.
“It would be great to see a systemic evaluation of mechanical sensitivity in human patients carrying these mutations. But the logistics of such a study would not be trivial, given the low number of these patients in different centers, and pain/mechanical hypersensitivity did not seem to be the main complaint for these patients,” he said.
Lincoln Tracy is a researcher and journalist from Melbourne, Australia. You can follow him on X – @lincolntracy.
References:
Sánchez-Carranza O, Chakrabarti S, Kühnemund J, et al. Piezo2 voltage-block regulates mechanical pain sensitivity. Brain. 10 July 2024.
Featured Image:
Sánchez-Carranza et al. Brain. 10 July 2024. Supplementary Fig. 15. Graphical Abstract. Model of how Piezo2 point mutations lead to nociceptor hyperexcitability.
Come discuss this article and more at Pain Researcher!