Opioids are a commonly used treatment to manage both acute and chronic pain, although there are significant fears regarding their potential for abuse. While the exact mechanism underlying the analgesic effects of opioids are not fully understood, the rostral ventral medulla (RVM) has been suggested as a key contributor since it’s the final relay nucleus involved in collecting and modulating information – including pain-related signals – from other regions of the brain and the spinal cord.
Now, researchers working in Patrik Ernfors’ lab at the Karolinska Institute in Sweden – led by Michael Fatt – have used single cell transcriptomics in combination with the manipulation of morphine-responsive neurons to identify that opioid analgesia is mediated by a subset of glutamatergic neurons in the RVM (which project to inhibitory neurons in the spinal cord) that decrease incoming pain signals.
“This work highlights the complexity between different neural circuits and cell types in mediating opioid analgesia. How and when these pain-inhibiting neurons are recruited under physiological and pathological conditions – and how these neurons interface with other known opioid sensitive circuits to produce behavioral changes – remain important questions,” wrote Caitlynn De Preter and Mary Heinricher in their perspective of the paper by Fatt et al.
The research and the accompanying perspective were published in Science on 30 August 2024.
Forming an ensemble
Ernfors, the senior author on the paper, has spent more than a decade trying to understand how different types of external and/or environmental stimuli are transduced and transmitted by sensory neurons and the spinal cord. While the role of the RVM in the processing of incoming sensory information has long been established, Ernfors was curious to figure out how the ascending and descending pathways converge to modulate pain perception.
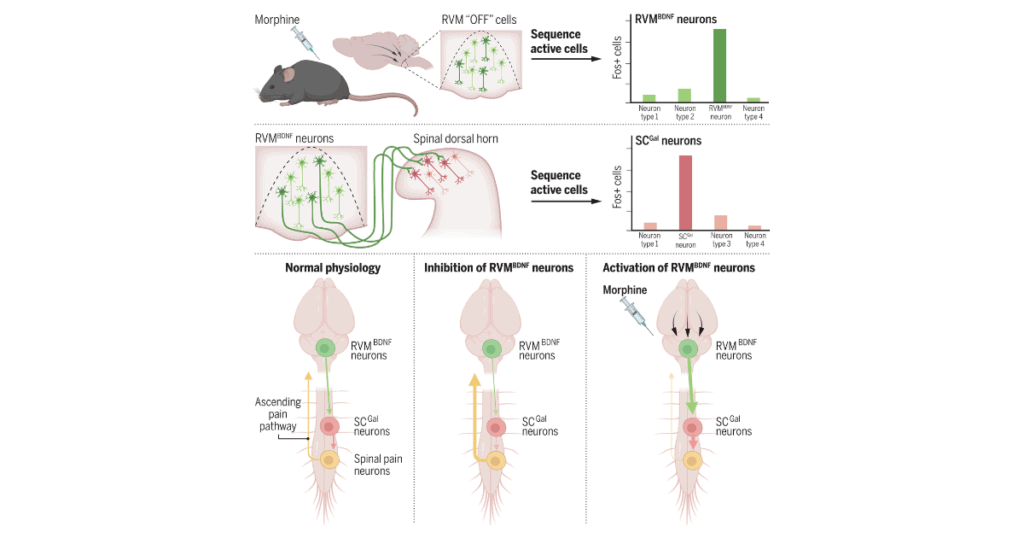
The first step in the process was identifying the collection of neurons within the RVM that were responsive to morphine, which was done by using stimulus-transcription coupling of the immediate early ARC gene. The activity of morphine-responsive neurons was synthetically increased or decreased by clozapine and salvinorin B prior to behavioral testing. This approach had not been used in the RVM prior to the current study.
“When you do the activity-dependent capture, you get an ensemble of cells, a lot of different kinds of cells [working] together. Some increase activity. Some decrease activity. But it’s still a network or a circuit of cells that we don’t exactly know ‘who’ is doing ‘what.’ [By] getting a molecular identity of the cells, we can suddenly start going into each of the different mechanisms and work out its functions,” Ernfors told PRF.
The molecular identities of the cells within the antinociceptive RVM ensemble were identified using single-nucleus RNA sequencing, with the process revealing 19 distinct neuron types. Four were glutamatergic, three were serotonergic, and the remaining 12 were GABAergic. Unsurprisingly, the GABAergic neurons were the most abundant, followed by the glutamatergic and serotonergic neurons.
Further testing revealed that the morphine-responsive antinociceptive ensemble was represented by changes in four key types of neurons: An increase in two types of neurons (the inhibitory GABA11 and the excitatory GLUT2) and a decrease in another two types of neurons (GABA9 and GLUT4).
Connecting the pieces
The team then developed a mouse model in which the morphine-responsive RVM neurons could be tracked to determine where these neurons project, and what happened when they were activated. Various techniques were used to identify that the RVM neurons projected to the dorsal horn of the spinal cord.
In line with the investigators’ earlier findings, approximately 60% of the RVM neurons were GABAergic, 30% were glutamatergic, and 5% were serotonergic (~5% consisted of different neuron types). Most of the GABAergic neurons projecting from the RVM were GABA10 neurons, which were unresponsive to morphine, leading the team to conclude that GLUT2 neurons accounted for the largest proportion of morphine-responsive output from the RVM.
This conclusion suggested that the GLUT2 neurons were the only excitatory projection coming from the RVM, and that this type was necessary for morphine-induced analgesia. These neurons are enriched with brain-derived neurotrophic factor (BDNF), and triple in situ hybridization confirmed RVMBDNF neurons played a key role in the morphine antinociceptive ensemble.
Ernfors was fascinated by the involvement of BDNF-expressing neurons.
“BDNF is a known trophic factor that shapes, or underlies, plasticity in the brain – like long-term potentiation. So critical aspects of plasticity in the nervous system are conveyed through BDNF,” he said.
However, the neural basis for morphine antinociception through spinal neurons had yet to be established. Single-cell analysis of spinal neurons taken from another specially developed mouse model revealed that the RVMBDNF neurons connected with galanin-expressing neurons in the spinal cord (SCGal). The SCGal neurons were later confirmed to play a role in morphine-based mechanical nociception.
“Morphine antinociception is elicited by the increased activity of RVMBDNF neurons that project to and activate GABAergic SCGal neurons. These SCGal neurons inhibit incoming mechanical pain signaling to reduce pain perception,” concluded first author Michael Fatt.
A potential role in hyperalgesia?
Ernfors and his collaborators used morphine in the current study to explore the complicated structure and function of the RVM because of morphine’s powerful effects on analgesia.
“The chance of actually getting all the different technologies that we use in this paper to work is greater if you have a model system with a robust effect size,” he explained to PRF.
But Ernfors is also interested in morphine due to its frequent clinical use, as well as the potential consequences (e.g., opioid-induced hyperalgesia) in cases where the drug is self-administered or used in a non-prescribed fashion.
Using morphine without properly adjusting the doses leads to a reduction in morphine-induced analgesia over time. The reduction in pain relief leads to people taking increased doses, which in turn leads to hyperalgesia, and before too long someone can persist in a state of hyperalgesia – regardless of how much morphine they are taking.
“That’s something we are very interested in understanding because it’s very likely mediated by completely different neurons in the RVM. So that’s something we’re trying to figure out – which neurons are inducing morphine hyperalgesia, and how this transition from analgesia to hyperalgesia is taking place,” Ernfors said.
While De Preter and Heinricher noted the current findings advanced our understanding of opioid-induced analgesia, they stopped short of declaring that the current study had identified a neural substrate for the analgesic effects of morphine.
“It may be more accurate to say that one neuronal population that can contribute to opioid analgesia has been molecularly characterized,” they wrote.
“The periaqueductal gray and the spinal cord have also been put forward as sites of opioid analgesic action because each site supports opioid analgesia, and blocking opioid actions at each prevents or reverses analgesia.
“Systematically administered morphine would naturally act in parallel at all relevant sites. Blocking opioid action at any one site prevents the analgesic effects of systematically administered morphine by eliminating the synergy among sites. Conversely, a high opioid concentration at any single site overcame the lack of synergy among sites, explaining the ability of focal microinjection to produce analgesia.
“It thus seems likely that other cell populations and circuits – in addition to those defined by Fatt et al. – contribute to opioid analgesia in most circumstances.”
Lincoln Tracy is a researcher and journalist from Melbourne, Australia. You can follow him on X – @lincolntracy.
References:
Fatt MP, Zhang MD, Kupari J, et al. Morphine-responsive neurons that regulate mechanical antinociception. Science. 30 August 2024.
De Preter CC and Heinricher MM. Opioid circuit opens path to pain relief. Science. 29 August 2024.
Featured image:
Graphical abstract. Identification of neurons that regulate morphine antinociception.
Come discuss these findings and more at Pain Researcher!