Delivering both innocuous and noxious stimuli to the rodent paw – primarily with von Frey hair filaments – is a canonical part of testing pain and mechanical sensitivity. Researchers often require extensive training before they feel confident in their ability to produce consistent results, but even experienced researchers can vary in terms of their consistency. This has led researchers to explore ways to automate stimulus delivery to produce more accurate and precise experimental results.
The latest approach to automated stimulus delivery comes from the Abdus-Saboor lab (Columbia University, New York, USA), which has designed a robot – the Automated Reproducible Mechanostimulator (ARM) – that is controlled using a video game controller and delivers mechanical stimuli to the paws of freely behaving mice more accurately, quickly, and consistently than even well-trained experts can.
“We’re in this heyday of computational neuroethology where we’re getting unprecedented ability to analyze animal behavior. If our behavioral output is getting better and better for analysis, we need to make sure our input is standardized as much as possible,” said Associate Teaching Professor Nathan Fried (Rutgers University Camden, New Jersey, USA), who was not involved in the current research.
“If we don’t standardize the input, what we end up getting is a lot of variability in the output – which is already very variable in terms of animal behavior. That makes it really, really difficult to interpret whether or not there’s going to be therapeutic potential in a new drug, for example.”
The preprint was published in bioRxiv on 7 May 2024.
Building the ARM
Joint first author Justin Burdge, a PhD candidate from the Abdus-Saboor lab, told Pain Research Forum that the idea of automating stimulus delivery in – and automating the analysis of – animal behavioral testing had been a goal since the lab was founded.
“We had been working on high-speed analysis of paw withdrawal behavior for evoked stimulus assays, and one of the things we realized was that our stimuli were all over the place. Sometimes the pinprick was very high, [but] sometimes it barely crossed the mesh at different angles and different speeds,” Burdge recalled.
“And [although] having those high-speed recordings allowed us to train ourselves better, to give more consistent stimuli, we never got to a point we were happy with. It still meant we were putting so much time and effort into training researchers before they could even produce usable data.”
The quest to automate stimulus delivery took a significant step forward when Burdge attended the Society for Neuroscience conference in November 2022.
“I was wandering around the exhibition hall after getting burnt out on too many posters [when] I discovered these linear stages a company was selling to use with microscopes and realized, ‘wait, we could use these to deliver our stimuli in a way that we could move it in x, y, z directions [and] even rotate it however we wanted.’ I began to imagine what we could modify and add to them to make this work,” he told PRF.
It took months of work with the mechanical engineering department at Columbia University following this “light-bulb moment” to transform this idea into the ARM. While some parts of the ARM haven’t changed since its original design, other parts – such as the 3D-printed stimulus holder – have undergone multiple iterations.
The ARM comprises three linear stages mounted and wired together with a stimulus holder (which can deliver cotton swab, dynamic brush, and pinprick stimuli) and a bottom-up camera to aim and deliver the stimuli from beneath the testing cages. The stimulus delivery aspect and bottom-up camera can be remotely controlled using a standard Xbox One controller. A second high-speed, infrared camera runs on a separate parallel track as an additional behavioral recording (e.g., paw withdrawal response).
Burdge claims to have been able to successfully train people on how to use the ARM in a little more than one hour.
“Unfortunately, that has led to a situation where I’m having difficulty booking the ARM to complete the [required] experiments for the final version of the paper, as it’s been fully booked for weeks,” he joked.
You won’t even notice I’m here
Habituating mice to experimental conditions (which previously included a researcher’s presence) is an important part of the behavioral testing process. The ARM significantly shortened how long it took to habituate mice to experimental conditions, reducing the time it took for the mice to come to rest (no turning, investigating, or grooming behaviors) over a three-day testing period.
Being able to speed up the habituation process is an important area of behavioral testing, according to Professor Jeffrey Mogil (McGill University, Canada). Mogil’s research focuses on social effects in pain behaviors in mice, but he was not involved in the current study.
“That’s a bigger deal than you would imagine, because one of the major frustrations for people doing preclinical testing in mice is just how long it takes them to stop moving. I like the idea of getting the experimenter out of the room, as there is always the potential confound of an experimenter being in the room, but [also because] the mice will actually calm down faster because they’re not anxious that there’s a big potential predator in the room,” Mogil told PRF.
“Anything that could get them to stop moving faster [means] you could end up doing more experiments per day per room, and that would be great for everyone.”
Anything you can do, I can do better
The ARM also significantly increased the accuracy of stimulus delivery, decreasing off-target distance of pinprick stimuli to a stationary target by 93% when it was pitted against five different researchers delivering the stimuli manually.
In further testing, Burdge and the Abdus-Saboor lab pitted the ARM against two external researchers with experience in delivering von Frey stimuli. The ARM was more consistent in how long it took to deliver stimuli (0% variation vs. a combined 61.2% for the human researchers) and was quicker in how long it needed to apply a stimulus to the mouse paw (50.9% reduction compared to humans).
Mogil was impressed with the spatial and temporal data of the ARM, but highlighted one area where he was slightly concerned.
“I’m a little bit worried about Figure 2E, because if you showed me those three stimulus-response curves and you asked me which is the best one, I would’ve picked the green one because it went convincingly over 50%,” Mogil said.
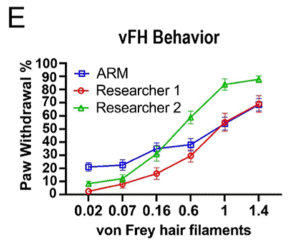
The stimulus-response curve in question, which looked at paw withdrawal percentage in response to different von Frey hair filament weights, belonged to one of the human researchers, who elicited a greater proportion of responses with higher weights compared to the ARM and the other human researcher.
However, Mogil acknowledged it was hard to say how worrying that result was (and where the variability is coming from), given the significant variability in 50% withdrawal thresholds and other algesiometry data he and his lab have observed over a 20-year period.
Fried echoed Mogil’s comments about the variability in the stimulus-response curves.
“It reveals [that] when you remove the experimenter from the room and you make the stimulus as consistent as possible, there’s still a lot of animal-to-animal variability [in how they respond]. What this says to me is that we need to measure more things with [respect to] the animal’s response, and we need to start figuring out what contributes to the variability,” Fried explained.
“That variability makes it really difficult for us to improve. It makes it really difficult for us to be confident on the discoveries we make in the pain field.”
Throw the kitchen sink at it
While Burdge was understandably tight-lipped about the changes the lab has made to the ARM since the preprint, he did highlight plans to start a program to get ARMs into other labs to help improve the design and make the technology more available to other researchers.
“One of our goals is to make a device that would allow a lab to jump into these experiments with no previous experience in doing them, which I think could be incredibly valuable in bringing new perspectives to the field,” he told PRF.
“[The ARM] also makes it physically possible for some researchers who, for one reason or another, cannot be standing for hours or bending over and targeting mice. We had one particular researcher in the lab in this situation, but once we had the ARM up and working, they [could] deliver stimuli [and] did experiments that ended up in the paper. I find that alone to be a fantastic result, even if it’s not the type of thing that often makes its way into the scientific literature.”
Burdge, together with Abdus-Saboor and other members of the research team, have filed a provisional patent for the ARM and founded a company which plans to commercially produce the ARM and other pain assessment software that would see them join other labs, such as Steven Prescott’s lab with their Reproducible Automated Multimodal Algometry (or RAMalgo) Robot, in making their product available for researchers to purchase.
Mogil joked he would have some level of distrust of any scientific evaluation of different automated stimulus delivery systems unless all parties were willing to give him, or someone like him, a free system to compare them in an external and unbiased fashion, but acknowledged this was unlikely to happen.
Fried had a similar view on things, saying, “We’ve got to throw the kitchen sink” at the ARM and other automated approaches to validate them in different settings, but conceded “that type of kitchen sink analysis takes a lot of time and a lot of effort, and that doesn’t always lead to a really sexy paper.”
Mogil told PRF that he felt the most likely scenario over the coming years would be waiting to see who starts publishing more papers with which system, and how many other labs take up (and continue to use) the various available automated delivery systems.
“At the end of the day it’s going to come down to some sort of cost-benefit calculation that individual labs are going to make about whether it’s worth going [through] all this trouble and money to make something that is already working so well work even better. I think a few years will pass and we’ll see who won just by seeing which one becomes the most popular. And that’s the way it should be, right? Let everyone get whichever system they think works best for them and we will see how democracy votes.”
Lincoln Tracy is a researcher and journalist from Melbourne, Australia. You can follow him on X – @lincolntracy.
References:
Burdge J, Jhumka A, Ogundare S et al. Remote automated delivery of mechanical stimuli coupled to brain recordings in behaving mice. bioRxiv. 7 May 2024.
Featured image: Burdge et al. bioRxiv. 7 May 2024. Figure 1A. Comparison between manual stimulus delivery that requires a researcher to aim and deliver stimulus by hand in close proximity to mice vs. robotic stimulus delivery via the ARM using motorized linear stages to maneuver and deliver stimulus and a bottom camera using a superimposed calibrated crosshair to aim.
Inline image: Burge et al. bioRxiv. 7 May 2024. Figure 2E. Both researchers and the ARM tested a cohort of wild-type mice (n = 10), applying each vFH 10 times to each mouse, producing the expected vFH response curves, includes SEM.


