Editor’s note: As the application cycle for the 2025 North American Pain School (NAPS) has opened, we’re taking a look back at some highlights from our PRF-NAPS Correspondents over the past two years.
Marco Loggia is associate professor of radiology and anaesthesia at Harvard Medical School (Boston, USA) and recently provided a plenary lecture at the IASP 2024 World Congress on Pain. Marco is particularly interested in the evaluation of neuroimaging metrics as potential biomarkers of clinical pain, and in the identification of brain alterations occurring in patients suffering from chronic pain conditions. In their quest to achieve a deeper understanding of the brain mechanisms of human pain, researchers in his lab use a variety of psychophysical and brain imaging techniques, such as functional magnetic resonance imaging (fMRI) – including blood oxygen level-dependent (BOLD) fMRI and arterial spin labeling (ASL) – as well as integrated positron emission tomography/magnetic resonance (PET/MR) imaging. Furthermore, Marco serves on PAIN’s Editorial Board as a pain measurement and imaging section editor. Here, PRF-NAPS Correspondent Emily Mills details Marco’s lecture from the 2023 North American Pain School titled, “From Brain to Joint: In-vivo Imaging of Inflammation in Human Chronic Pain.”
During this session, Marco Loggia presented his 10-year journey into understanding the role of inflammation in human chronic pain conditions. Throughout his lecture, Loggia interwove personal insights and anecdotes as a means of providing trainees with some career guidance – including how his journey into imaging neuroinflammation and peripheral inflammation in chronic pain began rather serendipitously.
A link between neuroinflammation and chronic pain?
Peripheral inflammation and neuroinflammation play important roles in initiating, progressing, maintaining, and even resolving pain in animal models of chronic pain (Grace et al., 2014; Fiore et al., 2023). However, until recent progress in human brain imaging methods, it has been very difficult to examine whether this is also the case in vivo in humans with chronic pain conditions. Since 2015, Loggia’s investigations have not only identified distinct neuroimmune profiles in different chronic pain conditions, but his most recent studies also point to the clinical utility of imaging these processes in humans.
Neuroinflammation and its role in the brain
Loggia began the session by addressing a simple yet important question: What exactly is neuroinflammation?
The term “neuroinflammation” refers to an inflammatory response in the nervous system, including the central nervous system (CNS; i.e., the brain and spinal cord). Unlike peripheral inflammatory responses, the main cellular players involved in CNS inflammation are glial cells – microglia and astrocytes. A neuroinflammatory response can involve the activation of these resident glial cells, the disruption of the blood-brain barrier (BBB), and sometimes the infiltration of peripheral immune cells including monocytes, macrophages, and T cells.
Albrecht et al., 2016 ACS Chem. Neurosci.
Loggia explained that neuroinflammation can be caused by many external triggers, including injury, infections, and toxic metabolites. It can also be caused by neurodegeneration, aging, and neural activity (termed “neurogenic” neuroinflammation).
“But is neuroinflammation a good or a bad thing?” Loggia asked. Similar to peripheral inflammation, neuroinflammation is an essential response that allows the nervous system to identify potentially harmful events; cope with pathogens, trauma, and toxins; and repair consequent damage. However, while this is a critical response in an acute context, persistent or exaggerated neuroinflammation is maladaptive and can promote further tissue damage, including demyelination, cell death and neuronal loss, iron accumulation, and edema (Albrecht et al., 2016).
Evidence for neuroinflammation in chronic pain: Animal investigations
So where does chronic pain fit into this?
Loggia described that, to date, there have been hundreds of experimental animal investigations that have identified a role for glial cells in models of chronic pain conditions. These investigations have shown hyperactive microglia in the spinal cord dorsal horn and throughout the brain in persistent pain states (e.g., Tsuda et al., 2003). Furthermore, a common marker of astrogliosis is increased expression of glial fibrillary acidic protein (GFAP), which is present in the brain and spinal cord in chronic pain models (Gao and Ji, 2010). Microglia and astrocytes can release pro-inflammatory cytokines to prolong persistent pain states, they can alter synaptic function, and lead to structural remodeling of neuronal circuits involved in pain processing (Grace et al., 2014).
Imaging inflammation in the human brain
Considering the vast body of evidence indicating a role for neuroinflammation in animal pain models, there has been a clear need to develop methods to image similar processes in humans in vivo.
“We have no real idea the extent to which neuroinflammation is relevant to human chronic pain conditions – because traditionally we have not had very good methods for imaging this,” Loggia explained, “And how can we know if a drug reduces neuroinflammation if we cannot even image neuroinflammation in the first place?”
Enter positron emission tomography (PET). PET is a powerful imaging tool that can measure blood flow, metabolism, neurotransmitters, and various other targets and mechanisms using radiolabeled drugs (or “radioligands”). In the context of imaging neuroinflammation, PET can be used as a means of visualising the expression of specific proteins which are found on glial cells as well as peripheral immune cells.
Loggia introduced the audience to the Translocator Protein (TSPO) – a marker of inflammation that can be imaged using PET with a radioligand known as [11C]PBR28 (Kreisl et al., 2010). Loggia explained that TSPO is a good candidate marker for neuroinflammation for several key reasons:
- It has a low basal expression in the healthy CNS but is upregulated by activated microglia and astrocytes.
- While TSPO’s “baseline” expression is not specific to any one type of glial/immune cell, its “overexpression,” which is observed in inflammatory responses, is co-localized with glial/immune markers in animal models of chronic pain (Wei et al., 2013).
- This is a protein we can actually image with PET. This makes TSPO an attractive marker when we attempt to bridge the gap between understanding neuroinflammation in both animal and human chronic pain investigations.
Neuroinflammation in chronic back pain and neurodegenerative conditions
Loggia reflected on his good fortune to have been involved in several studies during the latter half of his postdoctoral fellowship, which captured his interest in imaging neuroinflammation.
“Mine was a case of being at the right place at the right time,” Loggia explained. “Right at the time I was thinking about studying neuroinflammation in humans with chronic pain, new PET radioligands were being developed, and there was a lot of excitement about this at our institute.”
As he transitioned to becoming an independent scientist, Loggia began his initial PET studies in individuals with chronic low back pain (cLBP).
In his lab’s first investigation, he identified elevated TSPO in the bilateral thalamus in individuals with cLBP compared to pain-free controls who were matched for age and sex (Loggia et al., 2015). Importantly, these findings were remarkably consistent across individuals. “In imaging, it doesn’t happen very often where it is so clear who is a patient and who is a control by examining individual data,” Loggia explained.
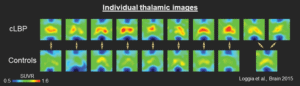
Upregulated translocator protein (TSPO) in the thalamus of patients with chronic low back pain (cLBP).
Loggia’s group replicated this finding across independent cohorts of individuals with cLBP (Torrado-Carvajal et al., 2021), which provided further support for these exciting results.
In subsequent studies, Loggia identified that elevated TSPO can occur in brain regions that are related to the pain specifically, whereas in other regions, TSPO is associated with pain-related negative affect. “An important theme emerging from the lab is that the functional significance of this signal depends on where you look in the brain,” Loggia described. “Not everywhere is related to pain – in some areas, the higher the depression symptoms, the greater the inflammation.”
Specifically, his group found that the neuroinflammatory signal in the primary somatosensory cortex is more pronounced in patients with greater degrees of “nociplastic” back pain – i.e., pain that is more centrally mediated (Alshelh et al., 2022). In other regions of the cortex that are involved in affective processing, including the anterior and mid-cingulate cortices, TSPO was more closely related with depression symptoms in cLBP (Albrecht et al., 2021).
Among other investigations, Loggia and his team also used [11C]PBR28 PET to identify TSPO elevation in several neurodegenerative conditions including multiple sclerosis (Herranz et al., 2016), Huntington disease (Lois et al., 2018), and neuromuscular disorders including amyotrophic lateral sclerosis (ALS) (Zürcher et al., 2015; Alshikho et al., 2016) and primary lateral sclerosis (PLS) (Paganoni et al., 2018). “In neurodegenerative disorders, there are clear hypotheses for where we might see neuroinflammation – and the elevation is exactly where we expect it to be,” described Loggia. These studies demonstrated the power of TSPO as a tool to image inflammatory processes and pathologies in humans.
Do different chronic pain disorders show distinct “neuroinflammatory signatures?”
Loggia’s next question was whether neuroinflammation in human pain conditions is unique to cLBP, or whether other chronic pain disorders also demonstrate neuroinflammation. “Did we get lucky? Is there something specific about pain in the back?” posited Loggia. “This can’t possibly be true, since cLBP is an umbrella term for many different pains.”
As the focus of his presentation turned toward other pain conditions, Loggia emphasized the importance of his collaborators. He explained how a collaboration with Eva Kosek at the Karolinska Institute in Stockholm began as a conversation at a conference poster session, in which they discovered that they were independently starting investigations to examine TSPO expression in fibromyalgia. “So at the beginning, we started doing a bit of a dance,” Loggia explained with a grin. They initially started sending each other emails back and forth to check how things were going with each other’s studies. “But at some point, we realized we should put our data together and see if we could find something reproducible in both sites. And we did, and I’m so happy we collaborated.”
Together, Loggia, Kosek, and their teams found that individuals with fibromyalgia exhibit elevated TSPO – but in very different areas from those with cLBP (Albrecht, Forsberg et al., 2019a). “This suggested that, first, neuroinflammation may be a mechanism involved in (and potentially a therapeutic target for) different pain conditions, and second, that maybe there are “neuroinflammatory signatures” that can differentiate patient populations,” he said.
Further evidence that TSPO PET signal elevation appears to be a rather pervasive phenomenon in chronic pain came from a subsequent investigation into Gulf War Illness – a multi-symptomatic disorder in which veterans experience cognitive and sleep issues, accompanied by musculoskeletal pain. In their cohort of veterans, Loggia’s group found elevated TSPO in areas similar to those identified in fibromyalgia patients (Alshelh et al., 2020). Gulf War Illness shares some overlapping features with fibromyalgia, and so this similar spatial pattern of TSPO upregulation paralleled the clinical similarities between the two conditions. Additionally, Loggia’s subsequent investigation also identified a distinct neuroinflammatory profile in migraine sufferers that correlated with the number of migraines experienced per month (Albrecht et al., 2019b).
Can TSPO also track inflammation outside the brain?
Whilst Loggia’s primary focus has been to study neuroinflammation inside the brain, there is overwhelming preclinical evidence for a role of inflammation in the spinal cord and peripheral tissues in chronic pain conditions.
This prompted Loggia’s group to investigate TSPO elevation in the spinal cord and neuroforamen (i.e., the part of the spine that contains the dorsal root ganglia and the nerve roots) of individuals with sciatica. They identified TSPO elevation in the spinal cord specifically at the levels associated with the affected nerves, as well as heightened TSPO in the neuroforamen (Albrecht et al., 2018). “In the neuroforamen, we can’t tell exactly where the signal comes from, as there is a mix of nerve roots and ganglia,” Loggia explained. “But we see greater TSPO signal in the foramen ipsilateral to the symptomatic leg, compared to the contralateral foramen, and healthy controls.”
However, he cautioned that this study should be taken with a grain of salt, given the small number of participants. He also noted that there was considerable inter-subject variability in the signal. “Maybe variability suggests that not everyone has elevated TSPO signal; this variability may indicate who will benefit from a local anti-inflammatory treatment and who may not.” Indeed, his team found that patients with elevated TSPO signal in the neuroforamen were those who experienced substantial relief with epidural steroid injections, demonstrating this approach’s clinical utility.
Their article recently published in PAIN reported that when Loggia’s team imaged TSPO in the joint of individuals with knee OA, they identified that those with a single knee affected show ipsilateral elevated TSPO signaling, and those with bilaterally affected knees exhibit bilateral elevated signal (Sandström et al., 2024). Furthermore, the signal is proportional to the pain intensity experienced.
“But wait! Glial activation in joints?” Loggia exclaimed. “Of course not!” He explained that, importantly, TSPO is also expressed by peripheral immune cells such as macrophages, monocytes, and many others – as was observed, for instance, in the joints of people with painful rheumatoid synovitis (Van Der Laken et al., 2008). As such, TSPO can be used to image inflammation both in the CNS and in the periphery.
Take-home message
Loggia’s 10-year journey into imaging inflammation in vivo in human chronic pain has yielded several key conclusions:
- TSPO shows promise as an imaging marker for neuroinflammation and peripheral inflammation.
- TSPO signal elevations can be observed across multiple pain populations, suggesting that immune/neuroimmune activation is a pervasive phenomenon.
- Inflammation/neuroinflammation may represent a therapeutic target for chronic pain (and related comorbidities), as predicted by animal studies.
Still, there are many questions that remain, and several clear next steps:
- There is a need to replicate this work in other research laboratories.
- We need to make neuroinflammation imaging more accessible – for instance, with magnetic resonance imaging instead of – or in addition to – PET imaging.
- It is important to disentangle the cellular components of the neuroinflammatory response with novel radioligands for distinct cellular players, such as microglia, astrocytes, and/or peripheral cells.
- We need to evaluate the anti-inflammatory effects of various treatments.
Take-home message for trainees
Loggia’s journey to image inflammation in chronic pain conditions began as a simple question that captured his interest. While he credits much of his journey and successes to lucky coincidences, many of the NAPS trainees in attendance, including myself, took this as an example of how motivation and dedication to pursuing a scientific question can lead us down a winding, yet fulfilling path. Early in his career, Loggia saw opportunities to get involved in related studies, even outside of pain research, and since then, he has continued to value collaboration over competition. His work is an example of the importance of following scientific interests and seeking out opportunities, even if the path forward isn’t necessarily clear at the time.
“Embrace being a bit lost,” Marco told us. “Don’t worry if you haven’t figured it all out.”
Emily Mills recently completed her postdoctoral research at the Krembil Brain Institute in Toronto, Canada. You can follow her on X/Twitter – @EP_Mills
References:
Albrecht DS, Granziera C, Hooker JM, Loggia ML (2016) In Vivo Imaging of Human Neuroinflammation. ACS Chemical Neuroscience 7:470-483.
Albrecht DS, Forsberg A, Sandström A, Bergan C, Kadetoff D, Protsenko E, Lampa J, Lee YC, Höglund CO, Catana C (2019a) Brain glial activation in fibromyalgia–A multi-site positron emission tomography investigation. Brain, behavior, and immunity 75:72-83.
Albrecht DS, Mainero C, Ichijo E, Ward N, Granziera C, Zürcher NR, Akeju O, Bonnier G, Price J, Hooker JM (2019b) Imaging of neuroinflammation in migraine with aura: A [11C] PBR28 PET/MRI study. Neurology 92:e2038-e2050.
Albrecht DS, Kim M, Akeju O, Torrado-Carvajal A, Edwards RR, Zhang Y, Bergan C, Protsenko E, Kucyi A, Wasan AD, Hooker JM, Napadow V, Loggia ML (2021) The neuroinflammatory component of negative affect in patients with chronic pain. Mol Psychiatry 26:864-874.
Albrecht DS, Ahmed SU, Kettner NW, Borra RJH, Cohen-Adad J, Deng H, Houle TT, Opalacz A, Roth SA, Melo MFV, Chen L, Mao J, Hooker JM, Loggia ML, Zhang Y (2018) Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients. Pain 159:968-977.
Alshelh Z, Albrecht DS, Bergan C, Akeju O, Clauw DJ, Conboy L, Edwards RR, Kim M, Lee YC, Protsenko E (2020) In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain, behavior, and immunity 87:498-507.
Alshelh Z, Brusaferri L, Saha A, Morrissey E, Knight P, Kim M, Zhang Y, Hooker JM, Albrecht D, Torrado-Carvajal A, Placzek MS, Akeju O, Price J, Edwards RR, Lee J, Sclocco R, Catana C, Napadow V, Loggia ML (2022) Neuroimmune signatures in chronic low back pain subtypes. Brain 145:1098-1110.
Alshikho MJ, Zürcher NR, Loggia ML, Cernasov P, Chonde DB, Garcia DI, Yasek JE, Akeju O, Catana C, Rosen BR (2016) Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology 87:2554-2561.
Fiore NT, Debs SR, Hayes JP, Duffy SS, Moalem-Taylor G (2023) Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol 19:199-220.
Gao YJ, Ji RR (2010) Targeting astrocyte signaling for chronic pain. Neurotherapeutics 7:482-493.
Grace PM, Hutchinson MR, Maier SF, Watkins LR (2014) Pathological pain and the neuroimmune interface. Nature Reviews Immunology 14:217-231.
Herranz E, Giannì C, Louapre C, Treaba CA, Govindarajan ST, Ouellette R, Loggia ML, Sloane JA, Madigan N, Izquierdo‐Garcia D (2016) Neuroinflammatory component of gray matter pathology in multiple sclerosis. Annals of neurology 80:776-790.
Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL, Zoghbi SS, Gladding RL, Jacobson S, Oh U, Pike VW, Innis RB (2010) Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage 49:2924-2932.
Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zürcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM (2015) Evidence for brain glial activation in chronic pain patients. Brain 138:604-615.
Lois C, González I, Izquierdo-García D, Zürcher NR, Wilkens P, Loggia ML, Hooker JM, Rosas HD (2018) Neuroinflammation in Huntington’s disease: New insights with 11C-PBR28 PET/MRI. ACS chemical neuroscience 9:2563-2571.
Paganoni S, Alshikho MJ, Zürcher NR, Cernasov P, Babu S, Loggia ML, Chan J, Chonde DB, Garcia DI, Catana C (2018) Imaging of glia activation in people with primary lateral sclerosis. NeuroImage: Clinical 17:347-353.
Torrado-Carvajal A, Toschi N, Albrecht DS, Chang K, Akeju O, Kim M, Edwards RR, Zhang Y, Hooker JM, Duggento A, Kalpathy-Cramer J, Napadow V, Loggia ML (2021) Thalamic neuroinflammation as a reproducible and discriminating signature for chronic low back pain. Pain 162:1241-1249.
Sandström, A, Torrado-Carvajal, A, Morrissey, EJ, Kim, M, Alshelh, Z, Zhu, Y, Li, MD, Chang, CY, Jarraya, M, Akeju, O, Schrepf, A, Harris, RE, Kwon, YM, Bedair, H, Chen, AF, Mercaldo, ND, Kettner, N, Napadow, V, Toschi, N, Edwards, RR Loggia, ML (2024) [11C]-PBR28 positron emission tomography signal as an imaging marker of joint inflammation in knee osteoarthritis. Pain 165:1121-1130.
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:778-783.
Van Der Laken CJ, Elzinga EH, Kropholler MA, Molthoff CF, van der Heijden JW, Maruyama K, Boellaard R, Dijkmans BA, Lammertsma AA, Voskuyl AE (2008) Noninvasive imaging of macrophages in rheumatoid synovitis using 11C‐(R)‐PK11195 and positron emission tomography. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 58:3350-3355.
Wei XH, Wei X, Chen FY, Zang Y, Xin WJ, Pang RP, Chen Y, Wang J, Li YY, Shen KF, Zhou LJ, Liu XG (2013) The upregulation of translocator protein (18 kDa) promotes recovery from neuropathic pain in rats. J Neurosci 33:1540-1551.
Zürcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE, Akeju O, Catana C, Rosen BR, Cudkowicz ME (2015) Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [11C]-PBR28. NeuroImage: Clinical 7:409-414.


