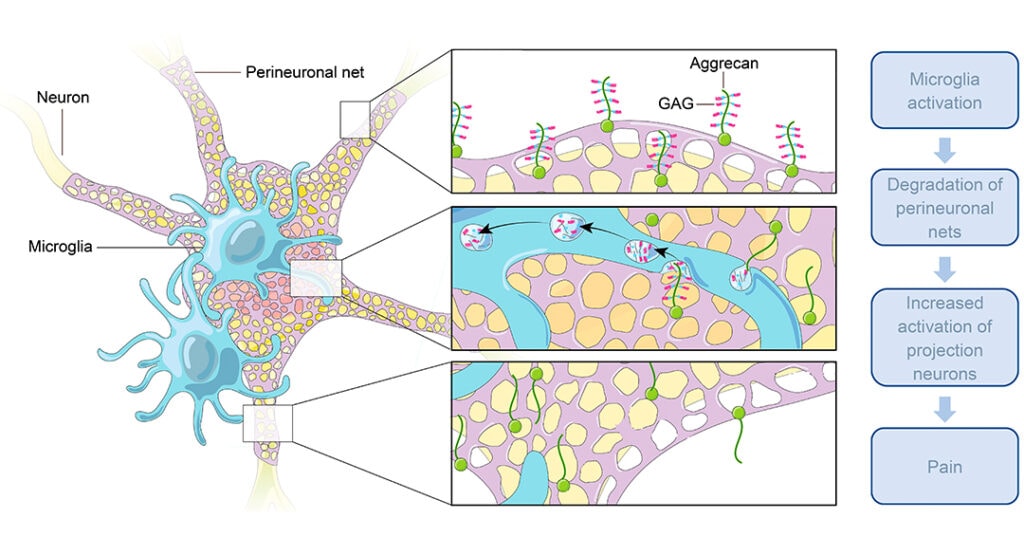
Perineuronal nets (PNNs) are basket-like matrices that surround certain neuronal somas in the cortex. They play important roles in the homeostatic regulation of neurons – controlling their plasticity and electrical excitability – and regulate critical periods of cortical development in early life.
But what are their roles in pain processing? Surprisingly little was known about these structures in the context of pain, but new evidence shows PNNs are important for the regulation of neuronal excitability in the spinal dorsal horn.
Work from the labs of Arkady Khoutorsky and Jeffrey Mogil, McGill University, Montreal, Canada, and additional collaborators shows that PNNs determine the background excitability of lamina I projection neurons in the spinal cord. However, after peripheral nerve injury, PNNs are degraded by microglia, causing disinhibition of lamina I projection neurons and neuropathic pain.
“This paper links two well-known spinal cord drivers of neuropathic pain – firstly, the involvement of microglial activation, and secondly, that increased synaptic excitation comes as a result of disinhibition – via a new mechanism: perineuronal nets,” said Ru-Rong Ji, Duke University, US, a researcher specializing in neuropathic pain who was not a part of the study.
The study was published on 1 July 2022 in Science.
PNNs surround neurons in the dorsal horn
“There has been a much larger focus on intracellular mechanisms and their involvement in neuropathic pain, but in our study, we wanted to focus on how modulation of the extracellular matrix is important in its development,” offered first author Shannon Tansley.
When Khoutorsky and his team began working, it wasn’t even clear if PNNs were found in the dorsal horn of the spinal cord. Khoutorsky, in collaboration with Luda Diatchenko (McGill University), had published a paper showing genetic evidence that the extracellular matrix in the spinal cord is regulated after nerve injury (Parisien et al., 2019).
As such, the team set out to visualize PNNs in the spinal cord of adult mice using immunohistochemistry. They found PNNs distributed throughout the spinal cord with a distinct localization pattern in the dorsal horn.
PNNs were enriched in lamina I of the dorsal horn, and retrograde labeling experiments found PNNs specifically clustered around neurons that sent ascending projections to the lateral parabrachial nucleus. This “spinoparabrachial pathway” is well known to be involved in nociceptive transmission and neuropathic pain (Basbaum, 2022).
“This led us to hypothesize that PNNs are important for regulating the plasticity of lamina I projection neurons,” said Khoutorsky.
PNNs weren’t just isolated to lamina I neurons; they were also scattered throughout laminas III-V, but were absent from lamina II. Counterstaining found that PNNs surrounded both inhibitory (Pax2+) and excitatory (Pax2- and NeuN+) neurons in deeper lamina.
The team decided to focus their attention on the lamina I projection neurons because of their role in sending sensory information to the brain.
“There aren’t many lamina I projection neurons, meaning a small subset of neurons has a strong effect on behaviors. We thought maybe these neurons need tighter regulation of their activity by PNNs, especially after situations like nerve injury,” said Khoutorsky.
Microglia degrade PNNs after nerve injury
The team was primarily interested in learning what role PNNs may have in neuropathic pain. Their first question was whether PNNs are modified after peripheral nerve injury.
To test this, mice underwent spared nerve injury (SNI) to model neuropathic pain, and PNNs were labeled in sections of the spinal cord at different timepoints following nerve injury. The team found that PNNs degrade around lamina I projection neurons as early as three days after injury and remained degraded for at least 14 days. Interestingly, only PNNs in lamina I degraded and not those in deeper lamina.
But what causes them to degrade? The number one candidate was microglia, which are known to be activated in the dorsal horn after nerve injury and degrade PNNs in the developing cortex to balance plasticity in neuronal networks (Takesian and Hensch, 2013).
Further immunocytochemistry experiments found that microglia contained glycosaminoglycan (GAG) fragments from PNNs (using wisteria floribunda lectin, or WFA labels) three days after nerve injury. Sixty-seven percent of all microglia in the dorsal horn contained these fragments, suggesting a major microglial component required to degrade the PNNs after nerve injury.
“It’s possible that microglial degradation of PNNs is protective. Early activation of microglia could have two actions: Firstly, that early inflammation is needed to ‘clean’ and protect the injury, and secondly, that microglia-dependent changes in the activity of the projection neurons are important for guarding/defensive behaviors,” hypothesized Tansley.
To show a more causal role of microglia in degrading PNNs, the team selectively depleted microglia in the spinal cord using genetic strategies. As expected, PNNs did not degrade after nerve injury in microglia-depleted mice.
“How do microglia degrade the nets? Do they release something to degrade them, or do they phagocytize them? We don’t know the mechanism yet, but we’re working on this,” said Khoutorsky.
Spinal PNNs are important for regulating nociception in uninjured situations
Lack of PNN degradation in microglia-depleted mice coincided with a lack of nerve injury-induced neuropathic pain behaviors. This led the team to hypothesize that PNNs may be key regulators of sensory transmission from lamina I neurons.
Specifically, they hypothesized that disrupting PNNs might induce pain, even in the absence of nerve injury. For this, the team once again utilized genetic strategies to ablate PNNs in the spinal cord, specifically those that project to the parabrachial nucleus.
The results were striking. Ablating PNNs caused thermal hypersensitivity in mice and spontaneous pain behaviors as assessed by the Mouse Grimace Scale. These findings suggest that PNNs may be important for the regulation of nociceptive transmission from lamina I spinoparabrachial neurons in normal, uninjured situations.
“This is an important finding in this paper. It suggests that PNNs are important for homeostatic regulation of lamina I neurons,” said Ji.
PNNs regulate disinhibition around lamina I projection neurons
As previously stated, PNNs in the cortex are known to regulate the plasticity of neurons by modulating their excitability (Takesian and Hensch, 2013). In principle, this shouldn’t be different in the spinal cord, but the team wanted to investigate how PNNs affect nociceptive transmission from lamina I projection neurons.
For this, they used electrophysiology to test whether the presence or absence of PNNs changed excitatory or inhibitory synaptic currents in lamina I projection neurons. Synaptic inputs were recorded using whole-cell patch-clamp recordings in an ex vivo spinal cord preparation. PNNs were degraded by adding chABC (chondroitinase ABC enzyme), a digestive agent that removes the GAGs that decorate PNNs and are known to play a role in synaptic function.
First, the researchers found that degrading PNNs makes lamina I projection neurons more excitable. Neurons had a depolarized membrane potential and an increased action potential firing rate.
Degrading PNNs also decreased the frequency of miniature inhibitor postsynaptic currents (mIPSCs), but not their amplitude. This means that lamina I neurons have impaired inhibitory synaptic input, thereby increasing their excitability. This disinhibition is well known to be a key mechanism of neuropathic pain, but what’s new is the evidence that degrading PNNs can play a role in this disinhibition.
In a final set of experiments, the team aimed to indirectly link disinhibition from PNN degradation with neuropathic pain. First, electrophysiology experiments showed that nerve injury caused a disinhibition of lamina I projection neurons. This could be prevented if microglia were ablated. When they degraded PNNs in microglia-depleted SNI-mice using chABC ex vivo, it brought back the disinhibition. Repeating these same experiments in mice, the investigators’ results were nearly identical to neuropathic pain, with degraded PNNs bringing back neuropathic pain in microglia-depleted mice after nerve injury. In summary, the degradation of PNNs, even in the absence of microglia, may result in disinhibition.
PNNs: The first step to cast knowledge out wide
Overall, this paper shows a novel mechanism by which microglial activation following nerve injury drives neuropathic pain: Degradation of PNNs. In addition, it also provides early evidence that PNNs have an important role in modulating the excitability and plasticity of dorsal horn neurons in adult mice.
According to Ji, the paper has opened up new ways in which we understand the mechanisms of sensory transmission and neuropathic pain, but he stated that there was a long way to go before it would have any therapeutic impact. For starters, Ji was curious about the role PNNs in deeper laminae would play in neuropathic pain.
“It’s logical that they focused on lamina I neurons due to their importance in pain signaling, but if you look at the data there are lots of PNNs in deeper laminae. From a therapeutic perspective, we also need to understand the role of PNNs in the whole spinal cord,” Ji told PRF.
Khoutorsky agreed, saying that this was one of many new questions the paper has opened.
“Is it possible to rebuild nets after nerve injury to alleviate neuropathic pain? What happens to PNNs after six months of nerve injury? Are they rebuilt? Is the same mechanism occurring in humans? These are just some of the important next steps that need to be answered,” Khoutorsky said.
Fred Schwaller, PhD, is a freelance science writer based in Germany.
Image credit: Designs that Cell