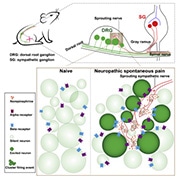
Spontaneous pain is notoriously difficult to treat. It can manifest in waves, seemingly at random, and is one of the most disruptive aspects of neuropathic pain conditions. In part, the intractability of spontaneous pain has come from a limited understanding of its underlying mechanisms, particularly compared to evoked pain. Several theories have been proposed about how spontaneous pain arises after nerve injury, ranging from the long-held idea that spontaneous pain arises from the ectopic activity of injured dorsal root ganglia (DRG) neurons, to the concept that spontaneous pain might actually be a form of summated allodynia and hyperalgesia from daily life (Bennett, 2012).
A new study from Xinzhong Dong at Johns Hopkins University School of Medicine, Baltimore, US, and Jun-Ming Zhang at the University of Cincinnati College of Medicine, Ohio, US, demonstrates that a peripheral nerve injury in mice can cause small clusters of DRG neurons to display ectopic activity. Furthermore, the authors show that sympathetic nerve sprouting into the DRG is responsible for this clustered ectopic activity, which is mediated by norepinephrinergic signaling. Blocking sympathetic activity or norepinephrine receptors had a powerful effect on reducing both ectopic DRG activity and spontaneous pain behaviors in mice.
“What’s important about this paper is that they’re putting the DRG as a source of neuropathic pain back onto center stage,” commented Marshall Devor of the Hebrew University of Jerusalem, Israel, who was not a part of the study. “The main idea here is that the DRG is a driver of neuropathic pain after nerve injury, and that sympathetic sprouting within the DRG amplifies that activity. The first person to show that nerve injury induces ectopic DRG neuron activity was Kirk in the 1970s (Kirk, 1974), and we showed how the sympathetic nervous system can enhance activity almost 30 years ago (Devor et al., 1994). So, while it’s an idea that’s been going around for some time, it’s nevertheless interesting and clinically important.”
The study was published in Neuron on 8 November 2021.
Clusters of ectopic activity emerge in the DRG after nerve injury
Dong and colleagues had been developing the method to use calcium imaging to measure DRG neuronal activation in vivo for some time. The technique has been used by several groups to measure activity in response to evoked stimuli; however, the authors wanted to investigate how spontaneous activity arises in DRG neurons after nerve injury, and how it relates to spontaneous pain.
The authors began by using the spared nerve injury (SNI) in mice as a model of nerve injury, and measured in vivo calcium responses in DRG neurons expressing the calcium indicator GCaMP6 21 days after injury. Almost immediately, first author Qin Zheng, who performed the experiments, saw that spatially secluded clusters of DRG neurons displayed waves of calcium responses in the SNI mice, without the need for stimulation.
“When we started recording un-evoked calcium signals in DRG neurons in anesthetized mice, it was quite remarkable. From the earliest experiments, we found that neurons fired spontaneously together in spatial clusters of about three to 50 neurons, not as individual neurons. This activity lasted for tens of seconds,” said Zheng.
Senior author Dong also highlighted how the clusters of ectopic firing activity didn’t happen very often – roughly four times per hour.
“Our calcium imaging setup was what allowed us to detect these waves of activity. It was important for us to image these DRGs for a long time, and have a way to image the whole DRG, to detect this activity reliably. This might explain why other groups haven’t seen the clusters before when using techniques like in vitro and in vivo electrophysiology,” said Dong.
Further probing with stimuli applied to the paw showed that these clusters of neurons contained a mix of nociceptors and low-threshold mechanoreceptors, but not proprioceptors. Moreover, this ectopic firing activity was only found following nerve injury, and not in inflammatory pain models.
Ectopic DRG activity as a source of spontaneous pain
The next question was how the ectopic activity in DRG neurons related to spontaneous pain. To test this, the authors measured spontaneous pain behaviors in mice 21 days after nerve injury. Most nerve-injured mice displayed jumping or flinching of the injured limb, indicating spontaneous pain-like behaviors. Next, the researchers performed calcium imaging in these same mice to correlate spontaneous pain behavior “scores” and the amount of ectopic activity in DRG neurons. As expected, ectopic cluster DRG neuron activity was more apparent in those mice that had these high spontaneous pain behavior scores.
They also found that not every mouse displayed spontaneous pain after nerve injury. Approximately 40% of mice did not display strong spontaneous pain behaviors, and this correlated with a low level of ectopic DRG activity in the same mice. These findings correspond with clinical presentations of neuropathy, where not every individual with neuropathic pain reports spontaneous pain.
Old ideas, new techniques, novel phenomena and mechanisms
A striking aspect from these initial experiments was that these clusters of ectopically firing DRG neurons were not randomly distributed in the DRGs, but rather near the nerve bundle entry or exit zones. The authors’ first idea to explain this phenomenon was that these clusters were mediated by gap junctions between DRG neuron cell bodies, but blocking gap junctions with carbenoxolone did not block the cluster of ectopic firing activity. Instead, the investigators turned to the locations of the clusters.
“This location reminded us of previous findings that sympathetic nerves innervate the nerve bundle entry zones after nerve injury. That’s how we made the connection between the ectopic activity and sympathetic nerve sprouting,” commented Dong, referring to findings from the 1990s (McLachlan et al., 1993).
After delving into older literature for inspiration, the authors turned to new tools to draw definitive links. Here, they investigated whether sympathetic nerves were indeed responsible for the ectopic DRG neuron activity in their experiments by using a sympathetic nerve-specific Cre-mouse labeled with tdTomato. The authors found substantial sympathetic neuron growth into the DRGs of mice with nerve injury, creating a link among sympathetic sprouting, clustered ectopic DRG activity, and corresponding spontaneous pain.
To show a more causative link, the authors inhibited sympathetic nerve activity to test whether this blocks the ectopic DRG neuron activity and spontaneous pain. They used three methods to inhibit sympathetic nerve activity: one pharmacological, via intrathecal application of 6-hydroxydopamine (6-OHDA); one genetic, by selectively expressing Gi-DREADD receptors in sympathetic nerves; and one by a very localized "microsympathectomy" (mSYMPX), performed by Zhang’s lab. All experiments yielded the same result: Inhibiting sympathetic nerve activity blocked both ectopic DRG neuron activity in vivo and reduced spontaneous pain behavior in mice. Moreover, activating sympathetic neurons, using a similar genetic method with Gq-DREADD receptors, enhanced the incidence of ectopic DRG neuron activity and exacerbated spontaneous pain behaviors in mice.
“It was curious that the clustering of DRG neuron firing wasn’t at least partly due to gap junctions. Here, the authors are pushing the sympathetic system as a mechanism, but it’s likely to be only one contributor. DRG neuron crosstalk is an important phenomenon associated with hyperexcitability after nerve injury, and there are three main hypotheses of crosstalk: potassium efflux exciting neighboring cells; neurotransmitter-mediated crosstalk; and gap junctions (Devor and Wall, 1990). It would be interesting to see how each of these phenomena are interacting with the sympathetic sprouting and noradrenaline release to drive clustered DRG neuron activity,” Devor told PRF.
Neuromodulation by norepinephrine
So far, the authors had shown convincing evidence that sympathetic neuron sprouting into injured DRGs drives ectopic activity in clusters of DRG neurons, which in turn drives spontaneous pain. These experiments had successfully corroborated earlier findings that nerve injury causes sympathetic sprouting into DRGs (Devor et al., 1994).
It was also known that sympathetic fibers release norepinephrine to amplify the ectopic activity of DRG neurons, and become a source of neuropathic pain after nerve injury. To test this, Dong and colleagues used genetic techniques to label the local presence of extracellular norepinephrine in the DRG. Here, expressing a norepinephrine sensor channel enabled the authors to measure the precise location of norepinephrine release in vivo using fluorescent imaging. Strikingly, norepinephrine release was observed in DRGs in nerve-injured mice in the areas where ectopic DRG neuron firing took place.
In a final experiment, the authors showed that blocking norepinephrine receptors in DRGs reduced the incidence of ectopic activity in DRG neurons following nerve injury. Specifically, α1, but not α2, receptor antagonists were effective at reducing ectopic DRG activity. Taken together, the validation of these findings using this entourage of advanced experimental techniques has created an opportunity to explore unanswered questions in the future.
“For me, this is a very exciting finding. Spontaneous pain is difficult to treat in the clinic, and our data give us a cellular handle to begin working on the challenge of treating spontaneous pain. Of course, there are still many open questions, but the techniques we used in this paper give us a way to test how different variables like analgesics or stress alter ectopic DRG neuron firing activity and spontaneous pain. It’s something we’re certainly interested in researching,” Dong told PRF.
Fred Schwaller, PhD, is a freelance science writer based in Germany.
Featured image: Graphical abstract. Zheng et al., Neuron. 2021 November; 110(2):209-220.