Cancer is a leading cause of death in North America, and extracts a highly damaging personal and economic toll on those who suffer from it. Many common cancers, including those of the lungs, prostate, and breast, metastasize to bone as the disease progresses, resulting in tumor growth and destruction. This process is often extremely painful, as over 75% of patients with advanced-stage bone cancer report experiencing moderate to severe pain. Even worse, this pain is often refractory to medications, including opioids. Two new studies give hope that patients may someday find relief through targeting of a novel immune regulator called STING.
Research led by Ru-Rong Ji, Duke University Medical Center, North Carolina, US, indicates that the activation of STING produces an immune response capable of both reducing bone tumor progression and pain through a complementary mechanism that acts directly on the primary sensory neurons that innervate the bone. In this way, STING activation could produce relief for patients and is also immediately translatable.
“Dr. Ji is really at the forefront of looking at cancer immunotherapies and whether they also have potential effects on the pain system,” says Yi Ye, New York University, US. “I think that STING is a really good example.” Though not involved in these two studies, Ye conducts research that aims to understand the neurobiology of cancer pain, with a focus on the translation of novel therapies for head and neck cancer.
These two studies were published January 13, 2021, in Nature, and July 27, 2021, in Nature Communications.
STING as an immune regulator
STING (stimulator of interferon genes) is a receptor localized on the endoplasmic reticulum of cells, and only recently discovered in 2008. STING belongs to the pattern recognition receptor family (PRRs), which are critical sensors of self- and pathogen-derived DNA.
STING activation leads to the induction of type-I interferons (IFN-I), namely interferon-α (IFN-α) and interferon-ß (IFN-ß). Type-I interferons are signaling proteins that disrupt viral replication and activate immune cells, such as macrophages and natural killer cells, and have been used with varying degrees of success to treat cancer in humans for nearly 40 years.
However, high-dosage interferon treatment often results in negative side effects, and is no longer a commonly used therapeutic. Instead, new therapies aim to modulate interferon production in a more nuanced fashion to treat cancer with the goal of producing fewer side effects. Since the activation of STING modulates this well-known anti-cancer pathway, it is perhaps not surprising that human clinical trials of several STING agonists are already underway for treatment of several types of cancer.
STING is highly expressed in dorsal root ganglion neurons
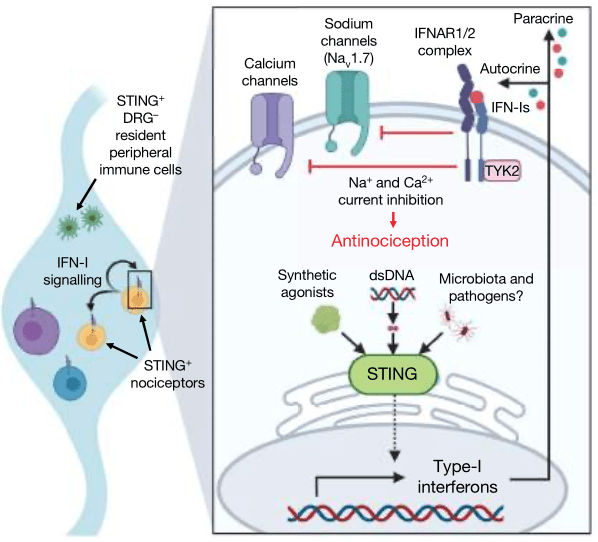
The authors began their investigation by studying a sequencing database of dorsal root ganglion (DRG) neurons – the neurons responsible for the transmission of somatosensory information from the periphery to the spinal cord. Specifically, the researchers combed the database for the expression of different PRRs, seeking to better understand how these DRG neurons can sense and respond to infection and injury (Donnelly et al., 2020).
During this search, they found that STING was one of the most highly expressed PRRs in DRG neurons, and that it was particularly enriched within peptidergic DRG neurons – well known to convey nociceptive information.
“I wasn’t surprised STING was expressed there because pattern recognition receptors are known to be expressed in sensory neurons, but I thought it was interesting that STING showed enrichment in nociceptive neurons,” says lead author of the first study, Christopher Donnelly, Duke University Medical Center, North Carolina, US. “I thought that was noteworthy and suggested STING may have a role in pain.”
Following this discovery, the authors administered STING agonists, including a clinically relevant agonist under investigation in Phase 2b trials, ADU-S100, to both naïve mice and a chemotherapy-induced peripheral neuropathy mouse model (CIPN). Although naïve mice were expected to serve as controls, the authors observed that intrathecal injection of each STING agonist significantly increased the pain threshold of these naïve mice as well as that of the CIPN mice. These experiments were then replicated in other chronic pain models, including the chronic constriction injury model and the spared nerve injury model. This provided the first evidence that STING is negatively regulating steady-state nociception.
Further experimentation using global STING knockout mice supported this finding, as lower pain thresholds were consistently observed. However, utilization of a global STING knockout model precluded the ability to determine which cells were involved in this steady-state nociception. To address this issue, the authors engineered another mouse model through which STING was conditionally knocked out from Nav1.8 expressing cells. Since Nav1.8, a sodium channel, is only expressed in DRG neurons, this model allowed the authors to determine if DRG neurons were the cells involved in this process. Utilization of this conditional STING knockout model resulted in lower pain thresholds and the decreased excitability of DRG neurons, allowing the authors to conclude that STING regulates basal nociception through its expression and activity within DRG neurons.
Elucidating STING activation pathways
To understand how STING could be regulating the activity of DRG neurons, serum and DRG tissue interferon levels were measured after treatment with STING agonists. The concentration of IFN-α was significantly higher in both the serum and DRG tissue collected from mice following treatment with STING agonists, and the basal production of IFN-ß was elevated in DRG tissue.
After observing that the stimulation of STING can increase interferon production in the DRG, in situ hybridization was utilized to probe for ifnar1, the RNA that codes for an IFN-I receptor component. Almost all DRG neurons possessed ifnar1, and moreover, a conditional Nav1.8 knockout of ifnar1 led to decreased pain thresholds. Finally, to determine how the induction of interferons by STING can lead to decreased DRG neuron excitability, the authors used electrophysiology to record from mouse DRG neurons. They observed that interferon perfusion led to a significant reduction in both sodium and calcium currents, and that this effect is abolished within ifnar1 knockout mice.
Following the combined analysis of their behavioral, in situ hybridization, serum/tissue sampling, and electrophysiology data, the authors concluded that the acute antinociceptive effects of STING occur through its activation in DRG neurons and the induction of interferon release, which then acts in an autocrine and/or paracrine fashion to stimulate interferon receptors on DRG neurons, ultimately leading to decreased DRG neuron excitability.
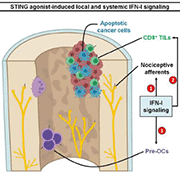
The three modes of STING action in treating bone cancer pain
Having demonstrated a direct effect of STING activation leading to a decrease in DRG neuron excitability, co-lead authors Kaiyuan Wang and Donnelly, Duke University Medical Center, North Carolina, US, conducted a follow-up study. Here, the authors investigated whether the direct suppression of DRG neuron activity by STING agonists could function synergistically with its previously known anti-tumor activity via innate immune system upregulation to reduce bone cancer pain and possibly even cancer progression.
The authors induced bone cancer pain through the injection of lung carcinoma cells into the femur of naïve mice. Mice were then administered an intraperitoneal injection of a STING agonist on days 3 and 7 following cancer cell injection. Behavioral tests indicated that these STING agonists were highly effective at reducing multiple measures of pain, including withdrawal threshold following von Frey stimulation and spontaneous behaviors such as flinching and guarding. Consistent with the findings of their first study, STING agonists were capable of relieving pain.
Next, the authors sought to determine whether STING agonists can also affect tumor progression. For this experiment, the authors used the clinically relevant STING agonist ADU-S100 and compared its efficacy to a common bisphosphonate bone cancer treatment, zoledronic acid (ZA). While both treatments led to decreased pain and tumor size, and increased locomotion in treated mice, ADU-S100 outperformed ZA in every metric. Moreover, the authors found that ADU-S100 reduced bone destruction and bone fracturing to a greater extent than ZA. Repeating these experiments within global STING knockout mice or global ifnar1 knockout mice failed to produce any alleviation of pain or bone destruction. Taken together, these results indicated that STING agonists are effective at both treating bone cancer pain and reducing cancer-induced bone destruction in a STING-initiated interferon receptor-dependent mechanism.
To determine if this improvement in bone cancer outcomes was due to the previously discovered DRG neuron-dependent mechanism, the authors turned back to the Nav1.8 conditional STING knockout mice, whereby STING was only removed from DRG neurons. In these mice, STING agonists failed to increase pain thresholds immediately following administration. After several days, however, these Nav1.8 conditional STING knockout mice treated with STING agonists had significantly less pain versus vehicle-treated mice. Furthermore, the reduction in tumor size was preserved in these Nav1.8 conditional STING knockout mice following treatment with STING agonists. This suggested that in this bone cancer pain model, STING can decrease pain progression through a DRG-independent mechanism, likely through the activation of anti-tumor immunity.
The authors utilized flow cytometry to measure the types of immune cells present in the bone tumor microenvironment and found that STING activation leads to an increase in tumor-infiltrating lymphocytes, which can destroy tumor cells. In a final, elegant set of experiments, the authors demonstrated that STING agonists also inhibit the differentiation of osteoclasts (the cells responsible for breaking down and reabsorbing bone), with no change in osteoblast (the cells responsible for producing bone) activity.
“The benefit of STING agonists is their synergistic protective effect when compared with opioids or bisphosphates. It will be helpful to test for potential pain relief effects of STING agonists in clinical trials,” says Wang, co-lead author of the second study. The authors believe it is this combinatorial effect that could make STING agonists particularly helpful in the treatment of bone cancer pain.
Translational potential
One of the most important things to consider with these two studies is their translatability. Not only do they utilize the STING agonist ADU-S100, which is already being used in human clinical trials, but additional experiments in the first study found a similar action of ADU-S100 in reducing DRG neuron excitability in both non-human primates and DRG neurons harvested from human donors.
However, there are some caveats to the use of STING agonists for the treatment of bone cancer pain. First, considerations must be made as to the clinical population. “There is data to suggest interferon signal decreases with age, and cancer actually happens more when people get older. So maybe there is an age-dependent effect for interferon/STING involvement in pain as well, and that could be something we could look at in the future,” Ye suggests, as a potential follow-up to further increase the translatability of this work to cancer patient populations.
Additionally, type-I interferon signaling has been demonstrated to be both anti-nociceptive as shown here, but also pro-nociceptive (Barragán-Iglesias et al., 2020). Indeed, in the first study presented here, the authors also found that the administration of high-dose interferon into the paw of mice produced transient pain. This makes sense, too, since interferons are activated by foreign pathogens like viruses, and many viruses are known to cause pain.
“We think infection is more complicated than just producing pain,” explains Ji. “It may also inhibit pain; it really depends on the stage of disease progression. Maybe there is some benefit for bacteria or a virus infection to inhibit pain.” He posits that our bodies may exist in homeostasis with regard to interferon signaling, whereby there could be an evolutionary benefit to inhibiting pain during infection or disease, especially in earlier stages when interferon activation may be only transient, and that STING agonists take advantage of this system to elicit pain relief.
This suggests that caution should be used when considering the dosage of STING agonists, but also for what pain condition it is being considered. “We don’t think that STING is going to be good for every type of pain,” says Donnelly. “In some circumstances, activating a robust immune response using STING pathway activators may be maladaptive. We just have to find the type of pain condition where the benefits outweigh the risk or cost associated with it, so that’s where we’re focused. We think cancer pain therapy is a really promising application.” For this reason, human clinical trials of STING agonists for pain relief may focus first on the most painful and intractable types of cancer, including bone cancer pain.
Erika Harding, PhD, is a postdoctoral fellow at Hotchkiss Brain Institute, University of Calgary, Canada.
Featured image: Schematic of mechanisms by which STING agonists directly and indirectly attenuate bone cancer-induced pain. Wang et al., Nature Communications. 2021 July; 12:4558.