A plethora of epidemiological studies demonstrate that chronic pain conditions are more common in women than in men. Women are also at a greater risk for developing common chronic pain conditions including migraines, low back pain, fibromyalgia, and irritable bowel syndrome, to name a few. However, the reason behind this is still poorly understood, although evidence suggests that a sexually dimorphic immune response might contribute to this phenomenon.
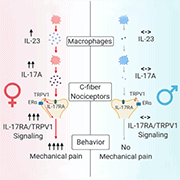
New research from Ru-Rong Ji, Duke University Medical Center, North Carolina, US, and colleagues shows that the IL-23/IL-17A/TRPV1 axis regulates female-specific mechanical pain via macrophage-neuron crosstalk. Interestingly, the sex dimorphism revealed in this study occurs at both the immune and neuronal levels.
Specifically, the authors demonstrate that: 1) Intraplantar injection of the inflammatory cytokine IL-23 induces mechanical pain specifically in females, but not in males. 2) IL-23 does not directly act on sensory neurons; rather IL-23 promotes the release of another inflammatory cytokine, IL-17A, from macrophages. 3) IL-17A activates nociceptors in mouse, monkey, and human dorsal root ganglia (DRG) neurons. 4) Low-dose IL-17A and capsaicin (a TRPV1 agonist) induce mechanical pain only in female mice. 5) Estrogen is essential in this process, as it enables IL-23 and IL-17A to produce mechanical pain via estrogen receptor α (ERα). 6) ERα expression by TRPV1+ nociceptors is required to induce female-specific mechanical pain by the IL-23/IL-17A/TRPV1 axis.
“… This study adds to a growing literature explicitly examining sex differences in pain, which is to be welcomed. A number of funding agencies now emphasize the need for inclusion of female test subjects,” wrote John M. Dawes and David L. Bennett, University of Oxford, UK, in an accompanying commentary. The commentary concludes in stressing that “not only do we need to identify sex-specific differences in preclinical models, but we also then need to consider the implications for sex differences in clinical trial design.”
“The Ji lab is well recognized for their work on neuro-immune interactions, and they found a new pathway through which IL-23 might be causing pain. Previous papers have shown a central mechanism of IL-23 in causing pain, and the Ji lab is demonstrating that there is a peripheral effect as well,” explains Thiago Cunha, University of São Paolo, Brazil, who was not involved in this research.
“This is a great paper that opens some possibilities into clinical therapies for pain using IL-17A and IL-23, as drugs targeting these pathways are already approved,” adds Cunha.
This research and accompanying commentary were published September 1, 2021, in Neuron.
IL-23 elicits female-specific mechanical pain in naïve mice
The authors first administered intraplantar injections of the pro-inflammatory cytokine IL-23 at various concentrations into male and female mice. They observed that von Frey testing of the hindpaw produced mechanical pain in females in a dose-dependent manner, but not in male mice. However, IL-23 did not affect the response of male or female mice to thermal stimuli.
When the authors repeated this experiment in IL-23 receptor (IL-23R) global knockout mice, the mechanical allodynia was abolished in females, which suggested that this IL-23 effect is mediated by IL-23R. Additionally, the IL-23R antagonist P2305 reversed this IL-23-induced mechanical pain in female mice.
To further investigate whether the IL-23 administration produced signatures of ongoing pain in female mice, the authors utilized a two-chamber conditioned place aversion assay. Following pre-conditioning, mice were pretreated with intraplantar IL-23 or vehicle followed by repeated stimulation with a subthreshold von Frey fiber to the ipsilateral hindpaw of both male and female mice. This stimulus is not normally painful in naïve mice but evoked a marked place aversion in female mice treated with IL-23, but not vehicle-treated females or IL-23-treated males.
What causes the sex dimorphism in IL-23-induced mechanical pain?
First author Xin Luo, Duke University Medical Center, North Carolina, US, next sought to understand what caused the sex dimorphism in this IL-23 signaling. To investigate, the authors utilized both pharmacological and surgical approaches to explore what role sex hormones might play.
When female mice were ovariectomized, IL-23-induced pain was largely prevented versus sham control mice. Conversely, male mice treated with estrogen subcutaneously now exhibited mechanical allodynia similar to wild-type female mice. This estrogen effect was mediated by ERα and not estrogen receptor ß (ERß), as subcutaneous treatment with an ERα agonist (PPT) but not an ERß agonist (AC186) enabled IL-23-induced pain in males.
The authors next explored why male mice were insensitive to IL-23. They found that androgen deficiency after male mice were orchiectomized enabled IL-23 mechanical pain versus sham operated mice. Similarly, androgen receptor antagonists enabled IL-23 to induce this mechanical pain phenotype in males. Conversely, subcutaneous testosterone prevented the IL-23-induced pain in female mice.
“Our results provide new insights into sex hormone regulation of pain. Using surgical and pharmacological means, we demonstrated that estrogen and androgen regulate IL-23-induced mechanical pain, which was mediated via ERα as well as androgen (testosterone), as it is able to suppress this pain,” explains first author Luo.
Do IL-23 and IL-23R drive chemotherapy-induced mechanical pain?
Luo and colleagues wanted to determine the role IL-23 signaling could play in chronic pain. To achieve this, the authors chose a chemotherapy-induced peripheral neuropathy (CIPN) model whereby the injection of the chemotherapeutic agent paclitaxel (PTX) produces neuropathic pain in both sexes.
Using the CIPN model, the authors collected serum and DRG from naïve and PTX-treated mice. They found that IL-23 was significantly elevated in the serum and DRG of the PTX-treated female mice; however, IL-23 levels in the serum and DRG from male mice were normal. This suggested that the PTX model results in both systemic and local upregulation of IL-23 specifically in females.
Next, the authors examined the mechanical pain elicited by PTX-treatment in knockout mice lacking either the ligand (IL-23) or receptor (IL-23R) of the IL-23/IL-23R axis. They found that both the IL-23 and IL-23R knockout reduced the mechanical pain in the early (day 3) and late phase (weeks 2-3) of PTX-treated females without changing the pain in the mid-phase (days 7-10).
In addition to this CIPN model, the authors also demonstrated similar female-specific mechanical allodynia effects of IL-23 and the IL-23R by using a chronic constriction injury model, a diabetic neuropathy model induced by streptozotocin, and a formalin-induced acute inflammatory pain model. The authors demonstrated that the IL-23/IL-23R axis is required for generating female-specific mechanical allodynia in neuropathic pain models as well as in the model of spontaneous pain caused by inflammation.
“Our findings provide new insights into the multi-level sex dimorphism arising from macrophage-neuron crosstalk during persistent states of pain. It is important to note that this is not a black-and-white (all-on-or-off) process, but rather this effect is graded at multiple steps along the axis,” explains Ji.
What are the key cellular players underlying the IL-23-induced mechanical pain?
The authors next investigated the cellular mechanisms underlying this IL-23-induced pain. Since it is well known that there are sex differences in the immune system response, and because IL-23 has been shown to be released by macrophages, the authors chose to utilize a mouse model where they ablated macrophages and monocytes via clodronate liposomes.
This ablation of macrophages and monocytes prevented IL-23-evoked mechanical pain. Furthermore, Luo and colleagues demonstrated that female macrophages displayed a larger IL-23 release in culture following PTX incubation versus male macrophages.
To further provide evidence that macrophages were specifically required for the mechanical pain phenotype in the CIPN model, the authors transplanted chemotherapy-activated macrophages between mice in a sex-matched manner and a cross-matched manner. They found that the transplantation of female macrophages was sufficient to induce more potent and persistent mechanical pain in female recipients versus male recipients. Conversely, male recipients exhibited greater mechanical pain when the chemotherapy-activated macrophages came from a male rather than a female donor, which supported this concept that the mechanical pain was indeed sex based and macrophage mediated.
When this experiment was performed using wild-type and IL-23 knockout mice, wild-type macrophages evoked sustained mechanical pain in both sexes, but IL-23 knockout macrophages produced less potent and persistent mechanical pain in female recipients. There were no differences between the mechanical pain induced by wild-type or IL-23 knockout macrophages in male recipients.
In support of the hypothesis that these sex differences were macrophage mediated, female DRG contained a larger subset of IL-23+ macrophages than male DRG under naïve as well as CIPN conditions.
It is well established that both C-fiber and A-fiber sensory neurons mediate mechanical pain through distinct mechanisms. In order to determine which subset is driving the mechanical pain observed in these models, Luo et al. turned to an ablation model of TRPV1+ sensory neurons. First, they ablated TRPV1+ C-fibers by resiniferatoxin injection and found that the mechanical pain induced by IL-23 was completely abolished. Next, they blocked C-fibers or A-fibers using QX-314 together with the C-fiber activator capsaicin and the A-fiber activator flagellin. They observed that blocking C-fibers, but not A-fibers, was able to prevent the IL-23-evoked mechanical pain in females, thus demonstrating that TRPV1+ C-fiber nociceptors are required for the IL-23-evoked mechanical pain in females.
This led the authors to next investigate whether IL-23 could directly modulate nociceptors. Using calcium imaging experiments, they directly applied IL-23 to dissociated DRG neurons and found that, even at high concentrations, IL-23 did not induce any calcium signaling in sensory neurons from either male or female mice.
Similarly, whole-cell patch clamp recordings showed that an IL-23 bath application did not alter the number of action potentials in response to a suprathreshold current injection.
“This was a bit surprising to us, and the most difficult connection we had to make was how IL-23 could cause the female-specific mechanical pain without IL-23 directly activating sensory neurons,” said Ji.
Data in the literature suggested that IL-23 signaling is upstream of IL-17A expression in T cells and macrophages. Therefore, the researchers investigated whether IL-17A levels were altered in macrophages from PTX-treated mice. Indeed, the authors found that IL-17A levels were increased in both male and female macrophages, and that IL-23R knockout suppressed these IL-17A increases only in female macrophages treated with PTX compared to their male counterparts. Additionally, the authors showed that IL-23 alone is sufficient to elicit IL-17A release from mouse macrophages.
“Our original hypothesis was quite a bit simpler: We thought IL-23 would directly act on nociceptors. It took us quite a bit of time to identify IL-17A as the missing [link],” added Ji.
IL-17A and the mechanical pain pathway
To determine if IL-17A is responsible for the female-specific mechanical pain observed in the PTX model, the authors first investigated whether IL-17A alone could induce mechanical pain in female and male mice. Intraplantar injected IL-17A was able to evoke mechanical pain in female mice in a dose-dependent manner, and produced only short-term (one hour) mechanical pain in males.
The co-intraplantar injection of IL-17A or IL-17A-receptor (IL-17RA) neutralizing antibodies with IL-23 blocked the IL-23-induced mechanical pain in females, demonstrating that the IL-17A and IL-23 pathways were indeed connected.
It is interesting to note that estrogen deficiency after ovariectomy prevented IL-17A-induced pain in females, whereas androgen deficiency after orchiectomy rescued IL-17A-induced pain in males. This demonstrates that IL-17A-induced mechanical pain is also linked to sex hormones.
Returning to the CIPN model, the authors demonstrated that IL-17A+ and IL-17A+/IL-23+ macrophages were increased under CIPN conditions, and that IL-17A levels were increased in DRG. This demonstrated that IL-17A production and release from macrophages is required for female-specific mechanical pain.
Because IL-23 did not directly activate sensory neurons, Luo and his colleagues wanted to determine whether IL-17A could directly act on sensory neurons. Incubation of low-dose IL-17A with sensory neurons evoked calcium influx in ~6% of females but not male sensory neurons. Additionally, IL-17A application during patch clamp recordings also demonstrated that the action potential firing frequency was only increased in sensory neurons from female and not male mice, thereby demonstrating a sex-dependent activation of sensory neurons via IL-17A.
Connecting the individual puzzle pieces: IL-17A, IL-23, TRPV1, and ERα
In order to connect the various pieces of evidence that IL-17A, IL-23, and TRPV1 were all required for the female-specific mechanical pain, the authors performed numerous experiments using genetic and pharmacological approaches to inhibit the pathway at various stages.
TRPV1 deletion abolished the mechanical pain induced by intraplantar IL-23 injection in females. Additionally, IL-23R antagonists did not reduce the PTX-induced mechanical pain in TRPV1 knockout animals, demonstrating that TRPV1 was required for the mechanical pain. Targeting TRPA1 failed to produce the same effect, suggesting that the IL-23 pathway was TRPV1 specific.
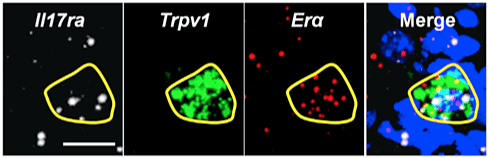
IL-17RA and TRPV1 are highly co-localized in ~40% of nociceptors in both male and female mice. C-fiber ablation and TRPV1 knockout transiently reduced the mechanical pain induced by IL-17A in female mice. Conversely, male mice exhibited less pain following intraplantar IL-17A, which was not affected after C-fiber ablation and TRPV1 knockout, suggesting that IL-17A signaling links to TRPV1 only in female nociceptors.
In a calcium imaging experiment, a small proportion of neurons were activated by IL-17A application, and these neurons also responded to the TRPV1 agonist capsaicin. Furthermore, the deletion of TRPV1 abolished the IL-17A-induced action potential firing in female DRG neurons, collectively demonstrating that TRPV1 and IL-17A are required for the mechanical pain in females.
But how does ERα fit into the picture?
IL-23-induced mechanical pain required ERα, but not ERß. When the authors investigated the expression levels of ERα, IL-17RA, and TRPV1, they found that ~10% of all male and female DRG neurons co-expressed all three receptors (with an average size of ≤20 μm, indicating that those were nociceptors).
The authors engineered ERα conditional knockouts, where the ERα receptor was specifically knocked out of TRPV1-expressing neurons. Both IL-23- and IL-17A-induced mechanical pain were completely abolished in the female conditional knockout animals. Estrogen administration enabled IL-23- and IL-17A-induced pain in littermate controls, but this effect was abolished in male conditional knockout animals.
Collectively, these data demonstrate that IL-23, IL-17A, TRPV1, and ERα are required for the female-specific mechanical pain.
“There are still a few questions that remain unanswered to me: For example, are other immune cells besides macrophages, such as g/d T cells or dendritic cells, involved in this IL-17A/IL-23-specific mechanical pain phenotype, as these cells can also release IL-17A? Additionally, the discussion surrounding a potential effect on the spinal cord and the activation by IL-17A of astrocytes is lacking, as the publication solely focuses on the peripheral effect of IL-17A/IL-23,” added Cunha.
Translatability of the IL-17A/IL-23 axis into humans
Since many preclinical studies on pain have not successfully translated into human models, the authors wanted to determine whether IL-17A could also activate nonhuman primate and human sensory neurons. A low dose of monkey IL-17A increased action potential firing in small DRG neurons from female rhesus macaques, but failed to do so in neurons from males.
In human samples, IL-17A was also able to increase the action potential firing in small human DRG neurons from both male and female donors; however, a lower dose of IL-17A only increased the nociceptor excitability from female DRG neurons.
Additionally, comparable expression of human TRPV1, IL-17RA, and ERα was found in both sexes, but higher ERα expression was observed in female DRG. Lastly, human plasma proteome profiles supported the translational relevance of this study, as IL-23 and IL-17A were found to be higher in females than in males.
“For me, one of the most important aspects of this study was to validate the findings in multiple different approaches such as pharmacological, genetic, imaging, and electrophysiological, as we wanted to be sure that the sex dimorphism at the neuronal and immune system level that we observed was truly valid,” stressed Ji.
“Here, we found that the sex dimorphism of capsaicin-evoked mechanical pain is promoted by estrogen in females and suppressed by androgen in males, implicating sex hormone homeostasis as a prerequisite for sex dimorphism in TRPV1-mediated mechanical pain. Of note, further investigations will be needed to explore the intracellular mechanisms underlying how the IL-17RA+/TRPV1+/ERα+neuron subpopulation mediates mechanical pain in females,” said Luo.
Notably, a follow-up study is being conducted by another investigator at Duke University Medical Center to validate this observation that IL-23 evokes mechanical pain in females. Furthermore, they plan to demonstrate that C-fiber activation-induced pain (e.g., licking behavior) can also be facilitated by IL-23 in a female-specific manner, using an optogenetic approach.
Ji and Luo agree that their research is important as they encourage the field to rethink the way we design and analyze data for clinical trials for chronic pain treatment, as it is likely that medications will not work equally in males or females. Additionally, multiple drugs are known to already target the IL-23 and IL-17A axis; therefore, the findings discussed here might encourage the field to re-examine the role of the IL-23 and IL-17A axis in chronic pain, which could lead to better targeted pain therapies for females.
Francie Moehring is a freelance writer based in Milwaukee, US.
Featured image: Working hypothesis. Luo et al., Neuron. 2021 Sep; 109, 2691-2706