Empathy – the adoption of the emotional and sensory state of others – is a fundamental social skill that helps people better perceive and respond to their external environment and to other individuals around them. This phenomenon is not unique to people, as studies have demonstrated the social transfer of fear, anxiety, and pain in rodents, too. Yet, while empathy has been extensively studied from a social psychology standpoint, its neurobiological correlates have remained elusive. What is happening in the brain that allows empathy and its associated behavioral manifestations to occur?
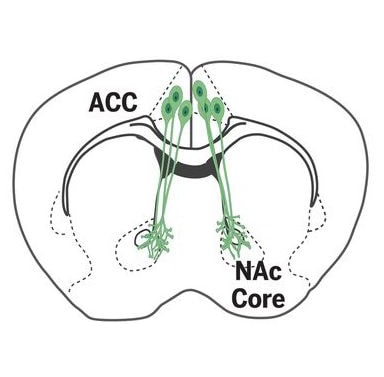
Now, new research from Robert Malenka, Stanford University, Palo Alto, US, and colleagues have identified a neural circuit involved in the social transfer of hyperalgesia, as well as pain relief, from one mouse to another. The researchers also show that this circuit, projecting from the anterior cingulate cortex (ACC) to the nucleus accumbens (NAc), is distinct from another circuit that facilitates the social transfer of fear.
Jeffrey Mogil, McGill University, Montreal, Canada, who first demonstrated social modulation of pain in a mouse model back in 2006 (Langford et al., 2006) but was not involved in the new study, said the research is “quite compelling.”
“The fact that one can define a neural circuit that is involved with this kind of social transfer, as opposed to pain perception itself, is very interesting – and something that many scientists would have doubted was possible not all that long ago,” said Mogil. “The fact that these researchers demonstrated two different ways this can occur suggests that the scientific community may have underestimated the prevalence of it, and in doing so, downplayed the importance of the social aspect in the biopsychosocial model of pain.”
In an accompanying Perspective, Alexandra Klein and Nadine Gogolla, Max Planck Institute of Neurobiology, Martinsreid, Germany, note that the demonstration of the social transfer of pain relief has clinical relevance.
“A deeper understanding of how and why analgesia can be transmitted socially may well have important future implications for pain management in humans,” they wrote.
The study and Perspective were published January 8, 2021, in Science.
Building on an “accident”
Monique Smith, first author of the new study, said that she first discovered the social transfer of hyperalgesia in animals “on accident,” while studying alcohol withdrawal in rodent models as a graduate student (Smith et al., 2017).
“I was looking at pain as a measure of alcohol withdrawal and noticed that the water-drinking control mice were also hypersensitive to pain,” she said. “At first, I thought I had made some kind of mistake and my control animals had somehow gotten the alcohol, too. But the more we looked into it, the more we realized that the withdrawn animal was actually transferring the pain behavior to other animals, and we learned that a brain area called the anterior cingulate cortex plays an important role. It was all quite serendipitous.”
When Smith came to Malenka’s laboratory as a postdoctoral fellow, the two decided to further pursue this line of research, using a variety of tools to better understand how the brain gives rise to what Malenka described as a “primitive form of empathy.”
“That early work of [Monique’s] gave us a window into how the brain allows us to feel empathy for another,” Malenka told PRF. “We decided to follow up with behavioral assays, optogenetics, and neural tracing studies to investigate the neural mechanisms that underlie these kinds of social transfers.”
The social transfer of pain
To begin, Smith and colleagues first developed a paradigm to rapidly transfer hyperalgesia to a bystander mouse housed with a cagemate. The cagemate received an intraplantar injection of complete Freund’s adjuvant (CFA) into a single hind paw. After injection, following just an hour of social interaction, the bystander mouse exhibited pain hypersensitivity in both hind paws (hinting at the involvement of higher brain regions) when tested with von Frey filaments. This hyperalgesia lasted approximately four hours. This was in contrast to control conditions, where the bystander did not exhibit hyperalgesia in the absence of a painful stimulus to the cagemate.
The bystander mice also showed thermal hypersensitivity in a hot water tail immersion test. And, like CFA animals, they preferred a floor at room temperature over a warm temperature floor in a thermal place test, whereas controls not experiencing pain showed no preference for either floor, indicating thermal place aversion. Further, an emotional discrimination task revealed that a “stranger” mouse placed in the cage spent more time sniffing a CFA mouse and a bystander mouse, compared with controls. This suggested that the bystander animals were experiencing an altered affective state from the social transfer of pain that could be detected by the stranger mouse.
From the ACC to the nucleus accumbens
In the next stage of the study, the researchers sought to identify neurons activated during the social transfer of pain, using a transgenic approach allowing for genetic access to neurons that are transiently activated by a stimulus. They found that neurons in brain regions like the ACC and nucleus accumbens (NAc), areas previously implicated in empathy and social motivation, were activated in bystander mice, as were neurons in regions like the thalamus, amygdala, and periacqueductal gray, which have been implicated in pain transmission. Interestingly, bystander mice had a greater number of activated ACC and NAc neurons than the CFA animals did. Neuronal tracing experiments would reveal direct synaptic connections between ACC neurons and activated NAc neurons during social transfer.
The researchers then used optogenetics to manipulate ACC neurons, or the ACC-NAc circuit, during the social transfer of pain. Inhibiting ACC neurons prevented bystander animals, but not CFA mice, from developing mechanical hyperalgesia. Optogenetic inhibition of ACC neurons that were activated during an initial social interaction also prevented mechanical hypersensitivity in bystander mice during a second social interaction.
Meanwhile, at the circuit level, inhibition of projections from the ACC to NAc during the one-hour social interaction impaired the social transfer of mechanical hypersensitivity in bystander animals, with no effect seen in CFA mice. Further, optogenetic activation of the ACC-NAc projections prolonged hyperalgesia in the bystanders. In this instance, the bystanders displayed hypersensitivity that lasted days instead of the four hours seen in the researchers’ initial experiments.
![Distinct ACC neural circuits mediate social transfer of pain states and fear. Complete Freund’s adjuvant (CFA)–induced pain is transferred from cagemates to bystanders after a 1-hour social interaction. Bystanders also exhibit pain relief after interacting with cagemates that are experiencing pain and morphine analgesia. The social transfer of pain and analgesia both require ACC-to-NAc projections, whereas the social transfer of fear requires ACC projections to the BLA [basolateral amygdala]. Data represent mean ± SEM; dashed line indicates mean baseline threshold for all groups; **P < 0.01 and ****P < 0.0001. Credit: Image and caption from structured abstract from Smith et al., Science. 2021 Jan 08; 371(6525):153-159.](https://www.iasp-pain.org/wp-content/uploads/2023/02/IFeelYourPainSlider4_0.jpg)
What about fear?
The researchers then wanted to see whether the same circuit might govern the social transfer of fear. Bystander mice showed increased freezing behaviors, indicating fear, during a fear conditioning phase where they could observe another mouse receiving footshock. This fear behavior was maintained a day later during a retrieval phase, where the animal was re-exposed to the context in which it had observed the other animal undergoing footshock.
But now, in contrast to the earlier experiments, optogenetic inhibition of the ACC-NAc projections during the conditioning phase had no effect on the acquisition of freezing behaviors; nor was there an effect during the retrieval phase. This was in stark contrast to the pain conditions, where the same animals showed impaired social transfer of pain upon inhibiting the projections. In the case of fear behavior, the researchers found that a different circuit, between the ACC and the basolateral amygdala, was necessary for social transfer of fear.
Finally, the team found evidence for the social transfer of relief from pain. In these experiments, all the animals received CFA, and then a portion of those also received morphine. It turned out that bystanders that had received CFA showed a reduction in pain in response to the von Frey filaments and in the thermal place test when they interacted with CFA animals that had received morphine. And here, too, optogenetic experiments pointed to the ACC-NAc circuit as a regulator of this behavior.
“We honestly didn’t know if that kind of transfer of pain relief would work,” Smith said. “And we were shocked that the behavioral output of the mice looks as strong as the morphine analgesia that the other animals received. This is something we’d really like to look at further.”
In their Perspective, Klein and Gogolla noted that a key outstanding question is how a single neuronal pathway can drive both the social transfer of analgesia and hyperalgesia.
“Perhaps different cell types are targeted in the NAc which affect distinct downstream brain regions,” they wrote. “Understanding this will be an important matter for future studies. Disentangling the circuits for social transmission more generally for positive versus negative affective states may improve our understanding of social and emotion disorders in humans.”
More empathy, better pain treatment?
Mogil said he would like to see further study of other circuits that may help facilitate these “robust and fascinating behavioral outputs” in the animals. He noted that the activation of cells in the insula would be an interesting place to look in future studies. And, like Klein and Gogolla, he hopes to see the emergence of a more detailed understanding of the differences between the positive and negative transfer states.
“The big question is how this transfer occurs, and what might be different between the heightened pain sensitivity and the pain relief,” he said. “The researchers suggest it might be olfactory, and finding the chemosignals that underlie this olfactory component would be very interesting.”
Malenka said he does not think the ACC-NAc pathway is the only circuit involved in what is an extremely complex social process. Yet he does believe that by elucidating these neural mechanisms in a mouse model, it may be possible to use that knowledge to understand and perhaps even increase empathy in human beings.
He also says the findings might lead to improved pain treatments, either by highlighting new potential drug targets, or by helping doctors and nurses find ways to better connect with chronic pain patients as they interact with them. He and Smith plan to keep looking at these social transfer circuits and test whether drugs that increase empathy, like 3,4-methylenedioxymethamphetamine (MDMA) – more commonly known as ecstasy or molly – may enhance the social transfer effects.
“I’m not an expert in pain,” Malenka said, “but as a physician, I could see that empathy was important in treating my patients from my first year as a medical student. So perhaps having an empathetic doctor could help with pain.
“Similarly,” he continued, “some patients find relief with group therapy. Maybe that is due to the empathic experiences that are shared in that setting. Or there could be a drug that might help. We just don’t know yet. There are a lot of detailed mechanistic questions left for us to ask here, and the neural mechanisms we discover should help us better understand the nuances of these quite powerful social experiences.”
Kayt Sukel is a freelance writer based outside Houston, Texas.