Twelve early-career pain researchers and clinicians are taking part in the second cycle of the PRF Virtual Correspondents Program. This science communications training program provides participants with knowledge and skills needed to communicate science effectively to a wide range of pain researchers and to patients and the broader public. Throughout the course of the program, the Correspondents will conduct interviews and podcasts with leading pain researchers, provide news and virtual meeting coverage – and blog posts! Take a look at their posts below, which will be published weekly over the course of the next six weeks.
Meet the PRF Correspondents

Week 6: Tuesday, December 8, 2020
The Relationship Between Chronic Pain and Sleep
Pain + Physical Medicine and Rehab? Let’s Team Up for Kids With Cerebral Palsy!
The Gap Between Research Output and Research Utilization
Art and Science – Are They Really Siloed Disciplines?
Impacts of the COVID-19 Pandemic Among Children With Sickle Cell Disease
The Extinction of Painful Memories
A Reflection From a “Scienthlete”
Global Public Health Messaging on Chronic Pain
Paywalls and Publishing: The Public Right to Open-Access Publishing
One of the hobbies I fell into during graduate school – and am still active in, to the extent that COVID safety measures allow – was circus, particularly aerial arts, like aerial silks, lyra, and trapeze. I’m still a complete amateur, but throughout my two or so years of taking classes at the wonderful Versatile Arts in Seattle (shout-out to my circus home!), one thing I have learned is that circus is often accompanied by a significant amount of pain. This is not only the typical muscle soreness of exercise (though there’s certainly that too – the first time I ever took an aerial silks class, my core muscles were so fatigued that it hurt to laugh or sneeze for days afterwards), or injury-induced pain, but also the pain of apparatuses. The very instruments that make circus possible hurt. I’ve gotten burns from rope and fabric, and bruises from the hard metal bars of a trapeze or lyra. Poses that from the outside can look like cozy lounging in a comfortable hammock of soft fabric often entail a lot of pinching of said fabric. This momentary discomfort is accepted, as long as it isn’t causing injury, and is even joked about – for example, I’ve seen shirts proclaiming “Circus hurts and so do I.”
Which led me to wonder: what is the particular relationship between pain and circus arts? Is there anything particularly unique about pain in circus? Is pain in circus somehow different (both physically and how it’s treated socially) than pain from other sports? Is there something about treading the line between acceptable discomfort and injurious pain that may be present in other sports but is particularly amplified in circus? Are there any prevalent myths about pain and pain management that circulate more in circus communities than among athletes of other sports?
My initial foray into the literature was not particularly enlightening. There is not exactly an abundance of papers that examine pain in circus, and most that do discuss pain only in the context of injuries and their treatment. For example, I found a few papers that examined the prevalence of injuries in circus performers, such as this study that analyzed a database cataloguing injuries in Cirque du Soleil artists. I also found case studies and conference proceedings that evaluated particular rehabilitation methods for certain conditions in circus athletes. One interesting, but very brief report detailed how pain levels correlated with stress levels in circus school students, but also left me with more questions than I had initially.
Many of the studies that referenced pain in the context of circus did so from a more sociological standpoint. I found sources that noted how some circus performers believed that pain was normal, pushing themselves to perform despite their injuries (a theme that seems prevalent in many sports, as other PRF Correspondents have noted in their blog posts). I even stumbled across a master's thesis in anthropology that, via interviews with students and instructors at an aerials studio, described how pain was often viewed as a “rite of passage,” a “necessary part of the journey,” and a kind of “initiatory ordeal” that had the power to forge connections between people and build community among aerialists. As a researcher who studies pain primarily at the level of nociception, I don’t often think about the concept of physical pain (as opposed to emotional pain or shared trauma) specifically as a way for people to bond, but I found this very relatable. My aerials classmates and I have certainly had frequent text message exchanges detailing our bruises, burns, aches, and pains; we’ve commiserated over the especially uncomfortable poses, like elbow hangs, and possibly grown closer for it. The author of this thesis noted that the storytelling of painful circumstances as a significant way to establish community is often quite specific to aerialists, but didn’t provide much evidence outside of their one field site; I would be very interested to see whether this is consistent across the circus community, or whether researchers have actually made comparisons between circus and other sports.
The basic, wet lab scientist in me is still somewhat dissatisfied with the results from my dive into Google Scholar – I longed for graphs, controlled experiments, studies that showed causation and not just correlation, and meta-analyses, which I slowly began to realize might be an unrealistic expectation for a rather niche topic. Perhaps one day other pain researchers will have the same sort of curiosity in this very specific topic. Until then, though, I’ll perhaps try to smile through the small painful moments in an otherwise very fun sport, and wonder if it has actually helped me find friends.
Kali Esancy, PhD, postdoctoral fellow, University of Washington, US.
You might be like the many other people (myself included) whose plant collection has exploded during quarantine. Working from home just seems a lot less lonely when you are surrounded by an expanding array of green foliage. Turns out I’m not alone in my appreciation of houseplants. As patients have explored alternative ways to manage their pain, some studies have emerged that suggest houseplants and nature help reduce a patient’s pain.
One study found that patients who were given house plants in the room where they recovered from appendix removal showed reduced pain, anxiety, and fatigue scores. In fact, patients don’t even need to be provided with a physical plant to benefit. In some cases, according to another study, patients who had a nature mural or sounds in their room also reported reduced pain levels.
The key to this puzzle most likely has nothing to do with some unknown magical property plants have to reduce your pain with a single glance. Most likely, looking at the plants in the patients’ rooms or the soothing nature pictures reduced their stress or anxiety. Maybe the nature scenes provided the patients with something to distract themselves from their discomfort.
Pain is far more than a physical symptom; it is tied closely to our emotions. People in a state of stress or anxiety about their pain, commonly referred to as pain catastrophizing, can experience more intense pain. This is why strategies that promote mindfulness or someone’s mental well-being, such as meditation, can effectively reduce that person’s pain. In a hospital setting, the plants or images could also help the environment feel homier.
Being in a hospital can be a scary and unfamiliar experience for many. The hospital décor often doesn’t help soothe these emotions. It’s possible that having some items in patient rooms makes the room seem a little more like home and helps the environment seem less foreign. This could help reduce patient stress and anxiety.
Some may think that all this talk about nature seems a little ridiculous. However, suppose something as simple and cost-effective such as having a couple of plants in hospital rooms or the homes of those recovering could reduce their pain and anxiety. In that case, I think it’s something that should not only be suggested but encouraged.
Courtney Bannerman, PhD student, Queen’s University, Canada.
The Relationship Between Chronic Pain and Sleep
As a chronic neuropathic pain sufferer, I am all too aware that chronic pain causes poor-quality sleep and frequent waking during the night; poor sleep is highly debilitating and I rank it my second most bothersome symptom (after pain). Sleep disorders are very common amongst chronic pain sufferers, with poor sleep quality and sleep disturbance occurring in 50%-90% of people with chronic pain. Interestingly, I have recently learned that the relationship between pain and sleep is bidirectional. That is, pain can disrupt sleep, but poor sleep can also predispose to chronic pain. In fact, there is evidence to suggest that sleep quality is a better predictor of pain than pain is of sleep quality.
It is intuitive to me that pain disrupts sleep. It has been suggested that chronic pain is the major reason for poor sleep (defined as difficulty getting to sleep, waking early in the morning, and feeling unrefreshed). It makes sense that when you are frequently woken up by pain, you spend far more time in the lighter phases of the sleep pattern and not enough time in the deeper sleep phases. When repeated through the night, this cycle results in a net sleep deficit and therefore consistent daytime fatigue. This has been confirmed in a meta-analysis that examined studies that utilized polysomnography (also known as a sleep study), which revealed that chronic pain is associated with less time asleep, delayed onset of sleep, fragmentation of sleep (due to more frequent awakening and movement-related sleep disruption), and more time awake after initially falling asleep. Sleep disorders are also exacerbated by many commonly used pharmacological therapies for chronic pain, including antidepressants and opioids.
What is not so intuitive (to me at least) is the other direction of the relationship – that is, the lowering of pain thresholds and increased spontaneous pain caused by poor sleep. Indeed, it has been shown that people with sleep disorders such as sleep apnea and restless leg syndrome are more likely to develop chronic pain. Likewise, studies in healthy volunteers have shown that sleep disruption causes increased pain sensitivity and a reduction in pain threshold. The underlying biological mechanisms(s) for this remains unclear, but is likely to include the opioid, endocannabinoid and monoaminergic (serotonin, norepinephrine, dopamine) systems, nitric oxide signaling, the HPA axis (hypothalamus-pituitary-adrenal), changes in the immune system, and melatonin. In a nutshell, sleep deficiency seems to deactivate systems that mediate analgesia, while also activating systems that mediate increased pain sensitivity.
Given this relationship between chronic pain and sleep, it has been suggested that medical management of chronic pain is more likely to be effective if concurrent sleep disorders are also treated. Indeed, a large-scale study demonstrated that short-term improvement of insomnia predicted long-term improvements in pain and sleep. It is surprising, then, that sleep disorders are often overlooked in the treatment of chronic pain, with half of chronic pain patients in a 2017 study being prescribed pain medications, and only 10-20% being prescribed sleep medications.
It has been suggested that management of chronic pain and sleep disorders requires a more individual assessment and treatment approach, and a shift from mainly medical management towards more psychological interventions such as cognitive behavioral therapy. More research is still needed to determine which treatments are most effective for the treatment of concurrent chronic pain and sleep disorders, so this is definitely an area to watch for those interested in the treatment of chronic pain.
Sherelle Casey, PhD student, University of Sydney, Australia.
Parents eagerly await the days when their children learn to walk, talk, and read. Baby books and tummy time are part of daily routines to encourage development of these important skills in the first months and years of a child's life. As a pediatric pain researcher, at times I dream up how we might begin to teach children about pain and its management as early as infancy. I imagine parents reading their child bedtime stories about learning how to communicate aches and pains. I envision story characters teaching that a comforting hug can reduce the unpleasantness of a scraped knee.
When I began to consider topics for my dissertation, it was important that it be a novel study. So I dove into an area of research that seemed interesting, and novel to me: mindfulness and pain. I was immediately enraptured by the way that attention and emotion intertwine with pain. I sorted through journal articles describing imaging the brains of Buddhist monks, and implementation of mindfulness interventions for inner city youth. And with each study that I devoured I excitedly thought I could conduct a cutting-edge dissertation study revealing that mindfulness could be the key to ameliorating the unpleasantness of pain for young children. I thought of the ways I would disseminate that finding to other researchers, parents and to children. Fast forward to the day that I started writing the literature review for my dissertation, and I realized I had a fabulously large pile of papers to summarize looking at exactly that research question.
Not only did I learn that research about mindfulness and pain is burgeoning, but I also found that there are hundreds of books for sale about mindfulness. However, most of these books are focused on chronic pain management. I have yet to find a published children's book that depicts a delightful story about a young child learning to approach their common childhood aches and pains with mindful attention. And whether parents would select such a book for their child's bedtime story over one teaching the ABCs is certainly unknown.
In my previous blog post I urged the pain research community to consider ways to creatively expand access, for the public, to cutting-edge pain research. In my final blog post of this invaluable experience as a PRF Correspondent, I am going to urge pediatric pain researchers to try to write directly to children. Children learn through story books, not journal articles. Now I imagine that publishing firms are not exactly reaching out to pain researchers to write a scintillating children's book about pain. However, if every parent wants their child to be a pain manager savant, perhaps that day will come. Because just as walking, talking and reading are essential life skills, I do think that learning about pain and its management can begin as early as a child's first baby books.
Wendy Gaultney, PhD, postdoctoral fellow, Oregon Health & Science University, US.
Pain + Physical Medicine and Rehab? Let’s Team Up for Kids With Cerebral Palsy!
As a pediatric psychologist, I provide care within a multidisciplinary pain team. We’re housed in a general outpatient clinic, sharing space with the physical medicine and rehabilitation (PM&R) team. They see a unique population of children, most commonly diagnosed with cerebral palsy. While PM&R and pain teams rarely share patients, I’ve been wondering if we should start.
What is cerebral palsy?
Cerebral palsy (CP; see here and here for more information about this condition) is the most common movement disorder in childhood and is a result of early damage to the developing brain. Symptoms and severity vary widely, including muscle stiffness, uncontrollable movements, and balance or posture problems. Recommended treatment is multimodal, ranging from medications, procedural interventions (Botox, serial casting, surgeries), bracing, and therapies (occupational, physical, and speech).
Do kids and teens with CP experience pain?
Although infrequently talked about, pain is a really common experience for individuals with CP! Two-thirds of youth with CP report experiencing acute pain in the past week and one-third report chronic pain. Muscle spasms and stiffness can be very painful. Posture changes and physical abnormalities can result in altered movement patterns that place stress on joints and muscles (this means more pain!). Kids with CP say familiar chronic pain triggers (e.g., too much or too little physical activity) can cause them more pain too.
Unfortunately, very common treatments for CP can also result in both acute and persistent pain. Approximately one-third of pediatric patients said wearing splints or braces caused them pain. One in four experience pain due to serial casting (repeatedly applying casts to slowly stretch contracted muscles and tendons). Over 15% also said therapies and Botox injections caused pain.
How do we address their pain?
Identifying pain, especially chronic pain, can be challenging as many children with CP also experience developmental delays, cognitive and/or communication disabilities. For those who are unable to talk about their pain, parents and providers can use behavioral pain measures (e.g., FLACC pain scale) and look for changes in daily activities to help identify the experience and impact of pain.
Botox injections are commonly used in the PM&R clinic at our hospital. Unfortunately, the anxiety and pain coming from patients receiving their Botox injections is palpable. There are many effective strategies to manage pain and distress related to needles (e.g., distraction, relaxation strategies, and comfort holds) that can easily be used when kids receive Botox injections. We know that repeated painful experiences can cause long-term changes to the body’s pain sensitivity, which would heighten the risk that a child with CP goes on to develop chronic pain. Pain can also cause patients to be less consistent in wearing their splints and braces, which makes them less effective. These medical devices should be trialed and modified when possible to ensure the most comfortable fit is reached. Finally, learning developmentally appropriate coping tools with the help of a psychologist can improve the self-management strategies patients and families use on a day-to-day basis to address pain.
It is clear to me that both acute and chronic pain are huge issues for kids and teens with cerebral palsy. There is room for growth in the identification and recognition of their pain as well as the use of well-known pain management strategies. A partnership between our pain team and PM&R is absolutely needed. It’s time to make a difference!
Mary Lynch, PhD, postdoctoral fellow, Indiana University School of Medicine, US.
The Gap Between Research Output and Research Utilization
When we meet a friend or a colleague after a long time, we generally ask them, “How are you doing?” or “How is life?” But now that we are all focused on research, we ask each other instead, “What’s happening with your PhD?” or “How many papers got accepted this year?”
Research productivity has been increasing exponentially over recent years as PhD and research output has become required for most academic positions. One recent study reported that the number of publications by Indian physiotherapists has increased from one in the year 1999 to 73 in the year 2018; I was happy to see that the university where I work ranked among the top universities in India with the highest number of physiotherapy research publications. With respect to chronic pain research, a simple PubMed search with the keyword “chronic pain” and filters (one year and randomized controlled trial) results in 95 hits.
PhD students are the key drivers of research output and the research trends that are posted over social media reveals that publishing 10-20 research papers in three to five years of one’s PhD has almost become a norm. One question that strikes me frequently is whether the end-user utilizes these large volumes of research to positively change clinical practice. A recent editorial published in the British Journal of Sports Medicine (BJSM) reported that clinicians primarily use “interactions with colleagues” and attend continuing professional development programs to change their clinical practice. This multinational survey of musculoskeletal and sports physiotherapists found that only 10% of the sample reported that they would read scientific articles to change their clinical practice. This clearly reveals that knowledge is not being adequately utilized by the end-user and significant efforts are required to achieve this objective of knowledge translation.
I would like to share one debate I have come across related to knowledge transfer between an ambitious researcher and a diehard clinician. The researcher has recently read about many good quality trials that say dry needling (the therapeutic use of thin needles) is ineffective for chronic pain and should not be used in clinical practice. The clinician argues that he does not believe in research conducted by others but uses his own clinical experience to guide his clinical practice; he found that dry needling has helped many patients with chronic pain. The clinician also referred to a lockdown YouTube talk delivered by a senior clinician who asked, “should I believe in the research conducted among only 100 participants by a therapist whose skills I am totally unaware of, or should I believe in my own expertise after treating 100,000 patients over the past 20 years?” This debate clearly validates the findings described in the BJSM editorial that clinicians are largely influenced by the practices of their fellow clinicians.
This is going to be a never-ending debate and I believe that similar challenges related to knowledge production and knowledge use exist across other pain specialties as well.
Finally, I discussed this issue with a friend to brainstorm some potential solutions to increase research utilization. After five minutes of discussion, my friend said, “Why is it always the researcher who has to inform the clinician about ideal practice, and why can’t it be the other way around?” These words turned my thinking upside down; maybe we can examine research methods and conduct research that further validates the clinician’s findings.
Y V Raghava Neelapala, PT, MPT, Assistant Professor, Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India.
Art and Science – Are They Really Siloed Disciplines?
In 2001, David Hockney published his seminal book, Secret Knowledge: Rediscovering the Lost Techniques of the Old Masters. Here he claimed, along with the physicist and expert in optics, Charles Falco, that the advances in realism in the history of Western art since the Renaissance were principally driven by the use of optical instruments such as the camera obscura and curved mirrors. The Hockney-Falco thesis is a controversial theory of art history, arguing that the realism and accuracy of Renaissance art was not solely down to the development of artistic technique and skill. Many interpreted the thesis as an accusation that the Old Masters intentionally obscured their methods, essentially “cheating.”
There is nonetheless a rich literature pointing to the widespread use of technology in the production of Renaissance art. The history of art and the history of the Renaissance is therefore also the history of optics. History has and continues to elucidate the enduring and intertwined relationship between art and science, a relationship that can be traced back to the Egyptian pyramids.
Why, then, do we still generally view art and science as siloed disciplines?
The Studio Lab of Art and Science (SLoAS) is a residency that brings together students from diverse disciplines; a contemporary example working at the intersection of the two fields, blurring the boundaries of understanding and invention in art and science. Exposing cutting-edge science to artists facilitates the translation of complicated concepts and novel technologies into art. On the other hand, participating in the artistic process exposes scientists to new and unique ways to think laterally, to approach problem-solving creatively, and to look at current research challenges in a different light. New insights can be gleaned from art-science collaborations that would not have been revealed by the artist or the scientist working alone.
Art is also a powerful yet underused tool for science communication and promotion of scientific engagement. The symbiotic collaboration of the arts and sciences is vital to fuel imaginations and spark debates; positive interactions between scientists and the public only build a more science-conscious populace, and a stronger coalition to eventually effect purposeful change.
The Wellcome Collection and Science Gallery London are two excellent examples of using art as a means of scientific outreach. Both are creating opportunities for people to think deeply about the connections between science, medicine, life and art through exhibitions and collections. The Wellcome Collection published a "stories" series last year titled "Painful realities," where Dr. Jaipreet Virdi wrote eloquently on how women’s pain has been viewed throughout the history of modern medicine, accompanied by artwork by Anne Howeson. The PAIN Exhibit is a valiant online visual art exhibition aimed at educating the public about chronic pain; it shows artwork that explores some facet of the pain experience of artists who suffer with chronic pain.
Synchronicity: pain research (and neuroscientific research more generally) seeks to understand the human condition, as does art.
C.P. Snow, the British chemist-turned-novelist, argued in his famous Rede Lecture at Cambridge University in May 1959, The Two Cultures, that the arts and sciences represent “the intellectual life of the whole of western society,” and the perceived dichotomy of the two disciplines is detrimental to the cause of furthering the progress of human knowledge. Since C.P. Snow mounted the podium in the Senate House in Cambridge to deliver his epochal lecture 60 years ago, there has been a narrowing of the gap between the arts and sciences through a multitude of programs and initiatives, of which I have mentioned a few. Nevertheless, there is still a gap between the Two Cultures that remains to be bridged.
Isobel Parkes, PhD student, University College London, UK.
Impacts of the COVID-19 Pandemic Among Children With Sickle Cell Disease
The impacts of COVID-19 are particularly salient for individuals with chronic conditions, such as sickle cell disease (SCD). One recent published study investigated changes in care delivery as a result of the COVID-19 pandemic in children with sickle cell disease. To examine these changes, the authors used the NHLBI (National Heart, Lung, and Blood Institute)-funded DISPLACE (Dissemination and Implementation of Stroke Prevention Looking at the Care Environment) study with the largest cohort of children with SCD. Impacts of COVID-19 were examined in 28 pediatric SCD centers part of the DISPLACE project and focused on transcranial Doppler ultrasounds, telehealth, chronic red cell transfusions and COVID-19 testing.
A total of 24 DISPLACE providers from the treatment centers participated in the study, and they reported 40% of patients as testing positive for COVID-19. The results revealed 92% telehealth usage with a combination of phone and video appointments. The authors stated that research on telehealth usage in patients with SCD is crucial in determining impacts on quality of care. Another finding was that, despite low blood supply concerns, most centers did not report difficulties with this issue. There were only a few cases where providers adjusted the type of transfusion in order to conserve the blood supply. Lastly, annual stroke screening, using transcranial Doppler ultrasound, was not significantly affected, with 67% of sites reporting being able to continue conducting screenings.
In conclusion, the authors said that, over the next 12 months, there will be a continued assessment of changes in care for individuals with SCD as a result of the pandemic. The authors suspected that the number of individuals infected with COVID-19 will likely increase since the initial assessment was done, making it imperative to continue to follow this situation.
Angela Pascale, PhD Student, Virginia Commonwealth University, US.
The Extinction of Painful Memories
Eternal Sunshine of the Spotless Mind is one of my favorite films. After a bitter break-up, the protagonists decide to erase their memories of their relationship. Erasing painful memories sounds appealing to forget your ex, but also in many medical contexts – for instance, forgetting a traumatic event or series of events that may have triggered post-traumatic stress disorder.
These examples relate to emotionally painful events, but what about physical pain? Acute pain is protective; it is an aversive sensation that motivates escape and triggers a strong learning experience. The phrase "once burned, twice shy" comes to mind. If a child touches a hot plate and experiences pain, they make the association between the action of touching the hot plate and the pain, and thus they are unlikely to ever intentionally touch a hot plate again in their lives. This strong learning experience of pain serves to protect an individual from re-experiencing the same injury again. However, in chronic pain, this protective function may have gone awry. In an insightful article, Vania Apkarian and colleagues suggested that ‘chronic pain is a persistence of the memory of pain and/or the inability to extinguish the memory of pain evoked by an initial inciting injury.’ The authors suggested that because the person is constantly experiencing pain, they make maladaptive associations between pain and an innocuous environment, such as their own home. As there is no respite from the persistence of their pain, these maladaptive associations are perpetuated in a positive-feedback cycle.
Extinction of memory is not forgetting but forming new memories to "update" the old ones. For instance, in an environment in which rats receive a shock, they will freeze, because they have learned the association between the environment and shock. However, when the shock is omitted, over multiple sessions the rats stop freezing because they update their memory of the environment from unsafe to safe. Extinction may be deficient in chronic pain patients for a number of reasons. One of these is explained by the fear-avoidance model. This model postulates that chronic pain patients may avoid certain things they have associated with pain (e.g., an activity such as shopping) even past injury healing. Whilst it’s better to be "safe than sorry" to avoid potential pain, this could prevent extinction of the pain-association memory, promoting the persistence of pain.
This theory could signal a big change in how we design future pain medications. Whilst currently we think of chronic pain as being a sensory disorder, with new treatments designed on this basis, thinking of it as an inability to ‘forget’ initial inciting pain could lead to a new group of medications. We might be far away from the memory erasure technology in Eternal Sunshine of the Spotless Mind, but cognitive enhancers that promote extinction have already been tested in rodent models of neuropathic pain and in chronic back pain patients, with promising results. I am excited to see how this field progresses.
Caroline Phelps, PhD, postdoctoral research associate, University of Arizona, US.
A Reflection From a “Scienthlete”
Here we are. At the last blog of this journey as a PRF Virtual Correspondent. At the last challenge (at least for now…) of bringing our matters of heart closer to you, PRF readers, in a creative, captivating manner. Was it easy? Admittedly, no. Was it worth it? Absolutely! Not only did I learn a lot during the writing process of my blogs, but I also enjoyed these moments of … I would call it self-reflection. To pause for a moment and to confront myself with the questions: Which topics really excite me? Why do they excite me? And how do I put this excitement in words to share it with you?
However, there are things I can't tell: whether you enjoyed reading my perspectives, whether I stimulated your own thoughts concerning the topics I discussed and whether some of it had or will have an impact – even if it's a tiny one. In my opinion, this is the ultimate goal of science communication: to leave a lasting impression.
In science, it happens too often that impressions don’t last – because of boring and incomprehensible means of communication. Luckily, there is proof of the opposite. For example, I will always remember a conference talk by Jeremy Narby. The Canadian/Swiss anthropologist and author is a project leader at a humanitarian organization, focusing on combating ecological destruction of the Amazon. In the talk I have come to enjoy, he discussed an Amazonian hallucinogen called ayahuasca and its therapeutic potential in trauma processing.
Narby's talk was scheduled for the late morning and I was getting a little drowsy in the dim light of the lecture hall. From the whispers and movements in the room, I could tell that other people were getting restless, longing for a break. Narby started his talk – without slides (and there would be none throughout the whole speech). “Quite unusual for a conference talk,” I thought, and prepared myself for another 45 minutes of troubles trying not to fall asleep. How wrong I was…his talk was absolutely mesmerizing. The lecture hall fell silent; people were hanging on his every word. His rhetorical skills made his speech come alive, painting a vibrant picture for his audience, needless of additional visual aids.
What can an early-career researcher learn from such an experience? Why was his talk so captivating? One could say it was an easy topic to talk about; hallucinogens are timely but controversial, kind of a forbidden fruit. But I think it was his passion – it was tangible. He really was a storyteller. And as Steve Jobs said: “The most powerful person in the world is the storyteller.” Stories bridge generations, inspire people's imagination and can broaden one's horizon. Science bursts with stories to be told – they just have to be told the right way.
With these thoughts in mind, I am sitting in front of my computer enjoying another moment of self-reflection. A moment of appreciation of this opportunity I was given to interact with the PRF community. To share my matters of heart. An attempt to learn telling stories the right way.
Thank you, PRF community, for this journey and of course, a big shout-out to my fellow PRF correspondents for their astonishing blogs. It was a blast.
Your honored "scienthlete,"
Laura
Laura Sirucek, PhD student, University of Zurich, Switzerland.
Global Public Health Messaging on Chronic Pain
The final PRF blog post is here! I hope you have enjoyed reading our thoughts and reflections as emerging pain scientists over the last six weeks.
I have been thinking about this final blog post over the last few weeks. The impetus for it came from my recent work synthesizing studies that evaluated the impact of mass media campaigns for chronic pain. We aimed to identify the key ingredients of successful campaigns and what a future chronic pain campaign would look like. In this blog post, I will share some of the key findings from our review and the importance of global messaging that targets everyone living with chronic pain.
What did we find in our review?
After evaluating 11 chronic pain campaigns (see the paper here), we found that most of them targeted back pain; of course, back pain is one of the leading contributors to global disability. The three common themes from our review were:
1. Targeted messaging: It’s important to find your “target audience” and your “key messages” and test them through various modes (e.g., television, print and social media). We found “staying active despite pain” as a common message across campaigns.
2. Cost versus reach: The relationship between campaign cost and potential reach was not straightforward. In other words, some of the most expensive campaigns had the highest reach, but even some of the low-cost social media campaigns had potentially similar reach. In fact, the use of social media as a primary campaign strategy was relatively less explored.
3. Impact: Studies that evaluated the impact of those campaigns suggested there was some change in pain beliefs but not necessarily in changing pain-related behaviors. One of the most interesting findings (at least for me) was that most of the campaigns were conducted in high-income, Western countries; we were unable to retrieve any campaigns or studies from low- to middle-income countries.
What would a global public health chronic pain campaign look like?
We know that chronic pain conditions like back pain and migraine contribute to disability worldwide. I have already discussed (see here) the sociocultural influences on chronic pain and the resulting inequities in adequate pain management. I am completely aware of the social, political, and economic influence of health systems globally, and they have a huge impact on the assessment and management of chronic pain. One of the key reflections from the COVID-19 pandemic is that consistent, global messaging on evidence-based information has helped mitigate the spread of the virus and misinformation. As the Director General from the World Health Organisation (WHO) said, “We are not only fighting a pandemic, but also an infodemic.”
A similar, concerted global effort by WHO, and also IASP in partnership with patient advocates, can help spread global public health messaging on chronic pain, which could be particularly important in raising awareness of chronic pain and help address myths about it, particularly in low- and middle-income countries. There is promising evidence that a targeted media strategy focusing on the Lancet Low Back Pain Series has helped to improve the accuracy of news stories reporting on back pain. However, the accuracy of those stories was low once the media campaign ended, and the lead author, Dr. Mary O’Keeffe, suggests we need ongoing efforts to provide accurate information. We are already facing a silent pandemic, as one in five people globally report chronic pain, and this (proposed) global public health initiative may improve chronic pain literacy around the world to help improve social narratives of chronic pain, promote help seeking, and ultimately defeat stigma.
Where to from here?
As I mentioned in my first blog post, we as pain scientists have an ethical and social responsibility to communicate pain science in order to counter misinformation, help address existing inequities, and improve social narratives. Ultimately, through meaningful partnerships with communities and people living with chronic pain, we can be “influencers” to make this invisible public health condition visible.
Hemakumar Devan, PhD, postdoctoral fellow, University of Otago, New Zealand.
Paywalls and Publishing: The Public Right to Open-Access Publishing
Scientific breakthroughs, new experimental drugs for fatal diseases, potential pandemic-ending vaccine trials for novel viruses, patient narratives, and clinicians as well as researchers critiquing healthcare policy to better their community and patients can all be found in medical journals. Yet similar to a gated community with signs of “No Trespassing Allowed,” many prestigious medical journals take research ownership and remain as gatekeepers, restricting public access to these resources and knowledge.
Public and patient partner voices have largely been absent from discussions about open-access publishing in medical research. But patients struggling to make informed treatment decisions, and nonprofit organizations advocating and lobbying for policy change as well as research funding, can greatly benefit from accessing scientific information quickly, easily, and most importantly, freely. I believe it is unethical to withhold such information from the public and, in fact, believe it should be a public right to have access to the medical literature.
The public plays a vital role in advancing healthcare policy and medical research by being advocates and serving as research participants, with the hopes that the results will be broadly disseminated for the collective good. The majority of academic research and knowledge published in closed-access medical journals is publicly funded, and, not surprisingly, most researchers who publish, as well as peer reviewers who review for closed-accessed journals, are based out of publicly funded institutions. Given that medical research is made possible through taxpayer funds, shouldn’t the public have full access to the research?
Open access is not only important for the general public to make their own decisions regarding their health and avoid misinformation, but also to determine where research funding should be allocated, for health journalists to report key findings, and for peer-to-peer information-sharing and collaboration among scientists to continue (especially those residing in low-income countries). Open access allows for public dissemination of knowledge, enhances transparency, can reconcile communication practices with patients, and may create meaningful public engagement opportunities. However, only 28% of all academic research is published in open-access journals. It’s not surprising that there’s growing frustration, within the scientific community (which conducts the research) and the public (the taxpayers who fund the research), with today’s outdated scientific publishing and business model that serve a small number of exploitative and exclusionary publishers by delivering them outrageous profit margins.
Universal open access should be viewed as a public health strategy to help educate the broader public. The COVID-19 pandemic has illuminated the benefits of, and accelerated the steady transition towards, the open access of scientific and medical research. With the entire world receiving open access to science without paywalls, it ensures that publicly funded science will inform taxpayers and reach nonprofit organizations for immediate implementation of evidence-based health precautions and further lobbying for policy change. As researchers, we must try to shift away from closed-access journals that deem the impact of our research solely on the basis of publication metrics like impact factors and citations rather than on the public availability of research that would support widespread clinical, policy, and social change. The impact, transparency, and accessibility of the research should be judged as more important than traditional publication metrics and the prestige of a journal. In the end, this will serve to preserve the quality of scientific research while increasing access for those who need it most.
Prab Ajrawat, MSc candidate, University of Toronto, Canada.
Week 5: Thursday, December 3, 2020
Is It All Over After the Age of 25?
If Exercise Is Medicine, Then Why Don’t Doctors Prescribe It to Chronic Pain Patients?
Preparing the Next Generation of Clinicians to Use Biofeedback
How Is Chronic Pain Managed in India?
Opioid Use in Children With Chronic Pain
Investigating the Comorbidity of Pain and Depression in Preclinical Research
Meaningful Partnerships to Address Inequities in Pain Management
Patient Partners in Pain Research
Is It All Over After the Age of 25?
“It all starts to go downhill after you turn 25,” a graduate student in the lab I worked in as an undergrad once told me, in a conversation that had somehow meandered to her husband’s recent aches and pains.
At first, I didn’t believe her. Twenty-five, after all, is not old. In some European countries, you still qualify for youth discounts on museum tickets and train fare; in the United States, you can’t rent a car without paying an extra fee. And as is so often proclaimed, your brain isn’t even fully developed until you’re 25.
But then, a few years into grad school, I turned 25, and I started noticing things. That I was stiffer and sorer than I used to be, that my back would sometimes protest if I spent a long time hunched over a microscope. I finally started to understand my dad, who frequently complained about shopping trips because of all the stationary standing it entailed, when my lower back occasionally ached as I stood by tables at science outreach events for hours at a time. At 26, I developed tendonitis in one knee. I’m not quite 30 yet, but whenever I bend down to pick something up, I feel like the human personification of Rice Krispies cereal – all snap, crackle, and pop.
Aging-related pain is nothing novel. We are peppered with references to it, from the personal anecdotes of friends and family to advertisements for analgesics to countless memes. Youths can seem impervious to so many physical mishaps, but when we’re no longer young, just sleeping the wrong way can wreck your entire day. As I’ve noticed my gradual increase in creakiness – and listened to similar complaints from my aging peers – the scientist in me has wondered: Why? Why do we, in general, have more aches and pains as we age? Do we literally deteriorate as we get older, and all of this accrued tissue damage subsequently activates our pain circuitry? Are older bodies just somehow more susceptible to pain-inducing injuries? Is it a matter of inflammation, or how our immune systems change as we get older? Is it something else entirely? Is it, as I halfway suspect, a compilation of multiple things, and that there’s no simple answer?
My initial searching rather depressingly suggested that physical breakdown likely accounted for a lot of the increases in pain – many doctors note that as we age, the connective tissue surrounding our joints breaks down, and becomes stiffer and less elastic. Inflammatory conditions like osteoarthritis, which are prevalent in older demographics, can hasten this gradual wear-and-tear. Even the discs in our spines, which act as shock absorbers for our vertebrae, become less “spongy” over time, setting the stage for herniations and other injuries that can cause pain. In some older adults, spines can even narrow with age, compressing and irritating nerves.
However, a deeper dive told me that this wasn’t the whole story. Many studies have shown that pain perception itself can vary with age, although the exact findings of individual reports are variable and often contradictory. An interesting meta-analysis of numerous studies found that, paradoxically, experimental pain thresholds increase with age, suggesting a diminished sensitivity to low-intensity pain despite increases in overall soreness. However, like some previous analyses, it also concluded that these changes in pain threshold are stimulus-specific, most dramatically affecting C-fiber mediated nociception, like thermal pain. Does this imbalance in sensory input or sensitivity as we age somehow contribute to heightened achiness? Still other studies have provided psychophysical evidence for alternations in endogenous analgesic/pain regulatory mechanisms as we age, as well as age-related differences in pain-evoked brain activity, particularly in regions involved in pain modulatory pathways. While the general wearing down of cartilage and the tissues that cushion our joints probably accounts for the bulk of our increased pains as we age (or at least that’s the medical consensus), I imagine that it’s plausible these other neurological factors, both peripherally and centrally, contribute to aging-related pain. But as almost every paper I perused stated, much more research needs to be done before drawing firm conclusions.
Having crested the age of 25, it may all be going downhill for me, a fact I am frequently reminded of when I lurch out of bed in the morning or creak my way down a flight of stairs. But there’s an odd sort of comfort in having even my superficial understanding of the mechanisms underlying aging-related pain, and I’m looking forward to what future research will reveal.
Kali Esancy, PhD, postdoctoral fellow, University of Washington, US.
If Exercise Is Medicine, Then Why Don’t Doctors Prescribe It to Chronic Pain Patients?
If there was a miracle drug that could lessen the pain for millions of chronic pain patients, with little to no side effects, you might expect policymakers to be all-in, pharmaceutical companies to produce it, physicians to prescribe it, and for every chronic pain patient to request it.
In fact, such a compelling treatment already exists: exercise.
For decades, treatment plans for chronic pain included recommendations for rest and inactivity to avoid pain flare-ups. However, there is now overwhelming and convincing evidence suggesting that exercise has a role in treating numerous conditions, including chronic pain (from my personal chronic pain experience you either move it or gain it!). A 2015 commentary in JAMA stated that “there is no medication treatment that can influence as many organ systems in a positive manner as can physical activity.” But if exercise is truly medicine, then why aren’t physicians talking about it or even prescribing it for pain management?
Exercise as a treatment is mentioned in almost all pain-related treatment guidelines, often written and consulted by physicians themselves. Physicians are also taught to discuss sensitive topics, including sex, bowel movements, mental health, and social relationships. Yet, patients are rarely counselled, screened, or asked about their exercise habits by their physician.
Part of the reason is that physicians themselves don’t inherently value exercise. Evidence shows that doctors are less likely to advocate for exercise when they themselves are inactive or overweight. Another reason is probably that physicians cannot bill exclusively for exercise counselling, at least not yet in Canada. A more important reason could be that physicians simply don’t feel comfortable or confident talking about exercise because they lack sufficient knowledge and medical training on exercise counselling. Western medicine, the current healthcare system, and medical education prioritize diagnosis and treatment while devaluing preventive and lifestyle medicine. This makes many physicians practicing today unfamiliar with exercise guidelines and they receive little, if any, formal training on the role of exercise in managing pain.
Current medical students and residents often report feeling incompetent when prescribing exercise but rate it as highly relevant. Exercise prescriptions are not yet prioritized at most medical schools nor in training in later years. However, this is about to change, with more medical schools incorporating exercise courses into their curriculums. By further integrating exercise counselling in clinical teaching environments, future physicians will not only understand the importance of exercise but gain clinical training on exercise prescription and counselling, as well as develop effective communication styles for motivating pain patients to exercise. Educating physicians that exercise is as good as many pain medications will be essential to using exercise as a first-line treatment and for holistically treating chronic pain. Just as the dose and frequency of pain medication are written down on a script, prescribing a “dose” of pain-alleviating exercise on a prescription pad is one simple and low-cost (it’s practically free!) way for physicians to help chronic pain patients reach their treatment goals.
I'm not advocating exercise as a cure-all nor am I saying that pain medications are a scam; I’m a physically active chronic pain patient who occasionally uses pain medication on tough days. But for me, exercise is a medicine that has helped me manage my pain and I believe it’s a safe, viable, and underutilized treatment for chronic pain. And, since physicians have an obligation to inform their chronic pain patients about the best-available evidence-based treatments, perhaps it’s time they get comfortable prescribing and talking about exercise for pain management.
Prab Ajrawat, MSc candidate, University of Toronto, Canada.
It was winter 2014. One day, I woke up with a sore throat and slight swallowing difficulties. But, well, cold season, ugly weather, hard volleyball practices – I was probably just a little tired and cold. Throughout the day, my throat got worse. At one point, swallowing became so painful that I decided to see a doctor. The doctor suspected tonsillitis and gave me antibiotics and acetaminophen, a very common painkiller for mild to moderate pain. I was relieved, went home to sleep and looked forward to getting better.
However, the opposite happened. My throat got even worse, I could barely eat and the pain got so bad, despite the painkillers, that I almost didn't dare to swallow at all (at this point: a cheer for banana milkshakes!). Since the antibiotics didn't work, I went back to the doctor, who now suspected a viral infection. Honestly, at that point I couldn't have cared less if a bacterium or a virus was causing the pain – I just wanted the pain to go away. Probably my desperation was very obvious, because I was given two other painkillers, one of them being tramadol. I was advised to take tramadol only if the pain became excruciating and to take a very small amount.
Was my pain excruciating? Hell, yes! So, I took the tramadol, with two consequences. First, I was knocked out. Second, once I woke up, I felt so sick that I had to vomit – not once, not twice, no, six times during two hours. Awesome! Did the pain go away? I don't remember, but as you can imagine, this experience really got stuck in my head.
Recently, I was reminded about this happening during the interview I performed as a PRF Correspondent (curious who I was interviewing? Check it out below!). At one point, we talked about opioids, and tramadol, which is a synthetic opioid, was mentioned. So, back in 2014, I was given an opioid. But it’s only now, after I knew about the opioid crisis and all the potential risks of opioids, that I have started to ask myself: Was it necessary to prescribe tramadol in my situation? Was I properly informed about the potential risks of this drug? Tramadol has a weaker binding potential to opioid receptors compared to other opioids such as oxycodone, so it is therefore considered to have a lower risk for addiction. Yet, recent evidence questions these assumptions and suggests that we be as cautious with tramadol prescriptions as with any other opioid (read all about it here). Another thought I had: would I have used tramadol again if I hadn’t gotten so sick?
Talking to friends here in Switzerland, I realized that my younger self is not an exception when it comes to limited knowledge about the risks of opioids. We rely on the knowledge of the medical personnel from whom we seek help. Can we always count on being informed properly? Are there options to gather trustworthy information ourselves if we're ever in pain?
I discussed these and other questions with Andrea Burden, a professor of pharmaco-epidemiology at ETH Zurich. Keep an eye out for the interview, which will be published on RELIEF, to check for her answers and to learn what pharmaco-epidemiology is and how it relates to opioids.
Your thoughtful "scienthlete,"
Laura
Laura Sirucek, PhD student, University of Zurich, Switzerland.
Preparing the Next Generation of Clinicians to Use Biofeedback
As part of my training to become a clinician-educator, I’ve been exploring teaching strategies and learning how to develop a curriculum. This had led me to dive into the literature surrounding the teaching of biofeedback in psychology programs.
Biofeedback is an incredibly helpful mind-body tool that gives you the ability to see changes in your body related to the stress response. Non-invasive sensors provide a visual display of things like our respiration rate, heart rate variability, and muscle tension. When we use biofeedback clinically, patients are able to see their everyday state and compare it to changes caused by relaxation strategies. Over time, individuals can learn to self-regulate without needing the visual feedback provided by the program.
This technology is particularly useful for individuals with chronic pain as it can help improve pain, symptom control and stress management. Biofeedback has been used across the lifespan and can be implemented in outpatient settings, inpatient programs, and can even be practiced at home. Biofeedback is included in the IASP curriculum for psychologists and is recognized by the American Psychological Association and the American Medical Association as a recommended mind-body therapy.
Many psychology graduate students are going on to work in academic medical centers as pediatric or health psychologists. As more patients are treated for chronic pain, graduate programs need to make training in biofeedback a priority. Unfortunately, the quality and depth of the biofeedback curriculum varies widely. A study of pediatric psychologists in North America found that only 30% received formal training in biofeedback and even less obtained certification. While a few universities have graduate-level courses, these are often supplemental and inconsistent. At the clinical internship level, practical experience is often provided during rotations but done with minimal didactics, simulations, or practice sessions.
There are no consensus statements regarding the recommended biofeedback training for graduate students. However for licensed professionals, the Biofeedback Certification International Alliance (BCIA) maintains strict educational requirements for certification in biofeedback. This includes courses on 1) anatomy and physiology, 2) science, history, and theoretical application, and 3) mentoring and supervision. After meeting these requirements, clinicians must also pass a certification exam and complete ongoing continuing education.
Formalizing the biofeedback training provided to psychology graduate students is important. Patients with chronic pain deserve the highest-quality care and the most effective treatment. In order to provide this, trainees should be given meaningful, in-depth, and well-rounded training in biofeedback. I suggest using recommendations from the BCIA, the Association for Applied Psychophysiology and Biofeedback, and IASP as guides to inform the didactics provided to students. Protocols for the different modalities (e.g., respiration, thermal, etc.) should be practiced through simulations, followed by supervised clinical practice. If graduate programs formalize their biofeedback training using established recommendations and publish their curricula, new pediatric and health psychologists will be prepared to effectively and efficiently help our patients with chronic pain.
Mary Lynch, PhD, postdoctoral fellow, Indiana University School of Medicine, US.
How Is Chronic Pain Managed in India?
I guess this should been my first blog post for PRF, but better late than never. India has a population of 130 crore (approximately 1.3 billion people) and, taking a minimum 10% prevalence of chronic pain, that leaves a whopping 13 crore (130 million) Indians suffering from chronic pain. That is a huge number that is more than the entire population of some countries.
But what is more interesting to ponder is how our health care system in India manages and treats these individuals. Prior to the COVID-19 pandemic, the opioid crisis was considered one of the biggest health burdens worldwide, but somehow India is an exception to this, since opioids are not usually prescribed for chronic pain here; many of us physiotherapists were not even aware of the opioid crisis until recently.
This blog post summarizes some of the pain management practices in India.
Optimists
A majority of chronic pain sufferers do not seek medical consultation in India. One survey found that 30% of the respondents (n = 5,000) did not take any form of treatment for their pain, and 40% felt that their pain was not severe enough to consult a doctor and that they had been managing to live with pain. Many of these patients think that pain is normal as one grows older and make up their minds to limit their daily activities. They would consult a medical doctor only when the pain is so debilitating that they are not able to perform those activities.
Medications and alternative treatments
Pain medications (prescription or nonprescription) rank first among the treatments received by people with chronic pain (56%), according to the national survey cited above. Interestingly, a considerable number of people (10%-40% in the survey) try alternative treatments such as ointments, herbal supplements, special oils, and gut cleansing juices. Pain medications and alternative treatments appeared to offer pain relief for an almost equal number of participants in this survey, which is fascinating. I was excited to see that 30% of participants tried exercise for their pain. If we extrapolate this to the entire population of Indians with pain, roughly four crore (40 million) Indians would be ready to undertake exercise for pain relief.
Traditional healers
Many Indians do believe in our traditional science that says pain or suffering can be due to karma (where past actions, good or bad, decide the future), or to improper vastu (the science of architecture). They usually perform rituals that are supposed to eradicate past sins or they have the vastu of their houses checked and corrected to relive their present suffering (in this case pain). Many others consult astrologers, gurus, and pandits for their pain, and there are many more practices I may not be aware of.
Opioid use in India
While some developed countries have experienced an opioid crisis, the Indian medical fraternity has clearly stayed away from prescribing opioids for chronic pain, thanks to our own unique ways of handling chronic pain, as described above. A recent review reported that the “medical use of opioids is very low and negligible” when compared to the rest of the world. The review cited several regulatory provisions in the Narcotic Drugs and Psychotropic Substances Act of 1985 as an important factor for limited opioid prescription in India.
So, this a snapshot of pain management practices in India. The key take-home messages: chronic pain is a huge problem in India; the majority of patients avoid medical consultations for pain and so are left untreated; and we need to work towards development of uniform and effective pain care.
Y V Raghava Neelapala, PT, MPT, Assistant Professor, Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India.
Opioid Use in Children With Chronic Pain
The relationship between opioid use and chronic pain treatment in pediatric patients remains an area of research with many unknowns. A recent article by Richardson and colleagues sought to address some of these unknowns by examining relationships between child and caregiver reports of child’s pain, physical function and socioemotional indicators with opioid use in pediatric patients starting chronic pain treatment. This is a particularly important relationship to understand as research has identified both short- and long-term side effects of opioid use. Further, opioid use over long periods of time may result in increased pain sensitivity and physical tolerance.
With these possibilities of side effects from opioid use, the authors recruited 1,155 pediatric patients aged 8 to 17 and one of their caregivers and, using binary logistic regression analyses, investigated how children’s clinical presentation impacts whether pediatric patients are prescribed opioids to treat chronic pain. Results indicated that the child’s age (i.e., older), pain duration (i.e., longer) and increased physical limitations (e.g, mobility challenges) were the most significant clinical correlates of the prescription of opioids. Interestingly, contrary to the authors’ hypothesis, socioemotional indicators such as anxiety and depression were not significant.
These results begin to elucidate our understanding of factors that influence whether pediatric patients are prescribed opioids to treat chronic pain. Understanding the factors influencing the decision to prescribe opioids in children is essential to prevent opioid abuse and dependence that may occur across the lifespan. The authors stressed that more research investigating these factors is needed, with the primary goal of better understanding the trajectory of opioid use and misuse.
Angela Pascale, PhD Student, Virginia Commonwealth University, US.
I have a lab mate who hates ticks with a burning passion. When we all went camping last summer, she came equipped with long pants, Skin So Soft®(a bath oil that seems to be an effective bug repellant), and knowledge of the most common places to find ticks. I, of course, knew about ticks as well and the diseases that they could carry. It wasn’t until I wrote about Lyme disease for my comprehensive exam that I realized how much was still unknown about the topic. Like many people, I was under the impression that Lyme disease was something that you would go to the doctor for to receive a course of antibiotics after seeing the classic bull’s eye rash. You’d feel a little unwell, but ultimately you would recover just fine.
For some people, this is the case, but for others, not so much. Some people can receive a standard round of antibiotics but still experience chronic fatigue, brain fog, and chronic pain long after treatment. Being a pain researcher, I was most interested in chronic pain present after a course of antibiotics. I completely understood why patients might experience pain before or during treatment, but after treatment was a little more puzzling. Did the antibiotics not work, or was there something more complex at play here?
Unfortunately, a search on PubMed didn’t reveal many studies looking at Lyme disease pain. Many studies were looking at the proportion of people who experience pain, among other symptoms, but why people experienced pain was a mystery that was still being worked out.
As someone interested in the interactions between the nervous and immune systems, it seems like this might be an area with some answers. The immune system would come into contact with the bacterium responsible for Lyme disease, Borrelia burgdorferi, early after infection. The presence of pain and other cognitive symptoms could signal that the nervous system is being affected. A growing body of research demonstrates how the nervous and immune systems interact to generate and maintain chronic pain in a variety of conditions. Maybe this could also be true for Lyme disease!
Sadly, like many others who live with chronic pain, Lyme disease patients can struggle to get their pain acknowledged by the medical system and often don’t receive adequate pain relief. When I was preparing for my comprehensive exam, I stumbled across more than one article belittling the pain that Lyme disease patients experience.
Regardless of our knowledge, or lack thereof, of Lyme disease pain, these patients are genuinely experiencing symptoms that make day to day life more difficult. They should be given the same respect and care that any other patient living with chronic pain would be given.
Hopefully, as research progresses, we will understand why Lyme disease pain happens and offer patients more effective treatments. As for my lab mate, she’ll continue to avoid tall grass regardless of what treatments are available.
Courtney Bannerman, PhD student, Queen’s University, Canada.
Investigating the Comorbidity of Pain and Depression in Preclinical Research
2020 … it has been a difficult year. The news has often been bleak, and as many countries impose a second lockdown to reduce the soaring numbers of new COVID-19 infections and death rates, isolation and economic anxiety are high. So, it is not surprising that there has been an increase in people reporting issues with depression. Yet, many chronic pain patients had comorbid depression in the pre-COVID era – an average of 52% of patients in a pain clinic setting compared to around 5% in the general population. How chronic pain may precipitate depression or vice versa is little understood but vitally important because patients who suffer from both disorders tend to have worse treatment outcomes than those with either disorder alone.
Rodent models could greatly aid in understanding the underlying mechanisms of this comorbidity. However, similar to behavioral assays of pain, many tests of depressive-like behavior in rodents have come under fire recently. One of the most popular tests is the forced swim test, which measures how long it takes for rodents to give up trying to escape from an inescapable water-filled tank. But this has recently been denounced as measuring stress rather than depression per se. Another way to look at depressive state is to measure anhedonia, the lack of pleasure in reward. In animal models the sucrose preference test is often used, in which rodents get a choice between a 1% sucrose solution and water, and a lack of preference for the sucrose is interpreted as anhedonia. It has been suggested that the sucrose preference test might also not be an effective test of depression, because there have been studies showing that patients with depression still can experience reward in the moment (which is what the sucrose preference test is measuring) but it is actually their memory of reward that is impaired.
During my PhD I used an assay developed in my supervisor’s lab to investigate how chronic pain might influence memory for reward. Rats were trained to dig in bowls, filled with different digging substrates, to find a sweet food pellet reward. On one day the rats had the choice between a blank substrate (e.g., wool) with no reward and a substrate rewarded with one food pellet (e.g., mouse paper bedding). On the next day the rats could dig in a different substrate rewarded with two pellets or the blank. This would be repeated twice, and then on the last day the rats would get a choice between the previously one pellet-rewarded substrate and a two-pellet substrate, which they would now see together for the first time. Even though both substrates were now rewarded evenly, control rats preferred the previously two-pellet-rewarded substrate because they remembered it as being more rewarding previously. However, the chronic pain rats performed at chance level, with no preference for either substrate. This was not a deficit in memory because the chronic pain rats learned the rewarded versus non-rewarded substrate in training sessions at the same rate as controls. This suggests that the chronic pain rats could not remember the different reward values of the substrates. This was interesting because subsequent tests in the lab showed that rodent models of depression also show this deficit, suggesting this could be a good assay for assessing depressive-like behavior in rodents and further investigating the comorbidity between chronic pain and depression.
Both pain and depression preclinical research have suffered from rodent assays that may not represent the human population that investigators are trying to model. Yet, with greater acknowledgement of this issue, and with new assays being developed and more widely used, there’s hope on the horizon. We just need to survive 2020 first….
Caroline Phelps, PhD, postdoctoral research associate, University of Arizona, US.
This weekend as I was washing dishes, I listened to the first episode of the podcast “A Matter of Degrees,” which is about the climate crisis. The co-hosts welcomed me as the listener and surmised that I likely was tuning in as a person who identifies as “climate curious.” The co-hosts shared their own journeys of becoming climate scientists/advocates. They then pegged me as a person who probably recycles, composts, and rides a bicycle to work (wow, spot on). I was intrigued by this classification of myself, the audience. I was further intrigued by the distinction between the co-hosts, who spend their careers on climate science, and me, a “climate curious” layperson.
During the past month of writing blog posts for PRF, it has occurred to me that I am also making general assumptions about my audience. My crystal ball says that you, my dedicated readers, are pain researchers, very likely my fellow PRF Correspondents, and perhaps have even experienced pain at some point yourselves!
Moving beyond the fact that I have now accurately identified you with my superpowers, I want to share with you what happened next in the episode of “A Matter of Degrees.” The co-hosts’ overarching message was to encourage me to stop expending my daily efforts toward reducing my own carbon footprint and focus instead on advocating for large-scale systemic change. While their message was empowering (and reduced my guilt about that glass jar I was too lazy to recycle), it also felt rather overwhelming. I am after all just a “climate curious” person who has a full-time job – I mean I am quite busy working to convince the world that pain is a big deal. I only tuned in to this podcast hoping to learn something new about the impending climate doom during my housework. I never meant to add to my to-do list an urgent email to my representative.
As I was reflecting on this message asking me to shift my own behavior in a way that supposedly may effect greater change, I realized that we struggle with this as pain researchers and clinicians as well. How much effort and resources do we expend on encouraging children with chronic pain and their families to make changes to their daily behaviors? Or how much do providers in clinical settings expend to manage pain better for their patients? And yet, there are clearly large systems that are clunking around in the background that are contributing to the problem of chronic pain at a much larger level.
Now, I am guessing that a podcast for the “pain curious” with the message, “Don’t worry about doing all of those things, instead, write to your representative about your concerns about how pain is managed,” may not be empowering. However, I do think that we would do well as pain researchers to take the time to consider our audience and our messages in ways that do effect change toward improved pain care. We may have to get more creative about how we think, and try a few different ways to reach our audience (like this amazing data visualization by my fellow PRF Correspondent Laura Sirucek) and then mobilize non-researchers who are concerned just as we are.
Wendy Gaultney, PhD, postdoctoral fellow, Oregon Health & Science University, US.
I am sure we are all familiar with the idiom “go with your gut,” and I suspect you are also well acquainted with the feeling of “butterflies in your stomach.” “Gut” as an adjective is often used to describe reactions that are instinctive and emotional; the relationship between the gut and the mind has long been intimately tied to our conscious understanding of emotions and embedded in our social discourse.
To illustrate, take a rather amusing example: in the 19th century, British doctors considered tea a nervous stimulant, and academic and public discussion alike was enraptured with the notion of excessive tea drinking. These discussions drew heavily on medical models of “nervous sympathy,” which emphasized the communication between the gut and the mind. In 1883, the Dean of Bangor, concerned with the quantity of tea being consumed in working class communities in North Wales, wrote in the North Wales Chronical (mind the hyperbole): “Excessive tea drinking creates a generation of nervous, hysterical, discontented people, always complaining of the existing order of the universe….”
The gut-brain axis began to capture our present-day collective consciousness with Michael Gershon’s popular 1998 book, The Second Brain. The enteric nervous system (ENS), or our “second brain,” comprises two layers of over 100 million nerve cells lining the walls of the gastrointestinal tract, with the gut-brain axis behaving as a bidirectional link between the ENS and the central nervous system. The gastrointestinal tract is home to a populous and intricate microbial ecological community and increasing evidence supports the existence of the microbiota-gut-brain axis, which has a not insignificant role in regulating the nervous system. The intricacies of gut microbial signaling via the gut-brain axis have been the focus of extensive research, and the gut microbiome has acquired cult status for its critical role in a variety of pathologies, including, but certainly not limited to, cardiovascular, metabolic, oncological, and neurological disorders.
Excitingly, there is an emerging role of gut microbiota as a novel regulator of pain, with a cornucopia of work suggesting that gut microbiota has a prominent role to play in visceral pain. In a paper published last year in PAIN, Minerbi and colleagues showed for the first time that the gut microbiota is also associated with chronic pain. Specifically investigating alterations in the bacteria in the gastrointestinal tracts of fibromyalgia (FM) patients, a syndrome most clearly characterized by chronic widespread pain, the authors observed a direct correlation between the abundance of several bacterial taxa and the severity of FM-related symptoms, including pain intensity and distribution. Disease-related variables explained this variance in the composition of the microbiomes between the FM patients and the control group more fully than any other innate or environmental variables. Expanding upon this, a LASSO machine-learning algorithm was able to classify with high prediction accuracy whether subjects were a FM patient or a control based on their microbiome composition alone.
It will be interesting to see whether dysbiosis (changes in the gut microbiota) is simply a biomarker of the disease, or whether it plays a causative role, and the extent to which dysbiosis is also correlated with other chronic, non-visceral pain, such as cephalgia or neuropathic pain.
Isobel Parkes, PhD student, University College London, UK.
Meaningful Partnerships to Address Inequities in Pain Management
As I mentioned in a previous post, I recently was invited to give a departmental seminar, and so yesterday I presented “Partnering with Māori (the indigenous population of New Zealand) whānau (families and significant others) to address inequities in pain management.” I shared my journey and reflections of engaging with the Māori whānau living with persistent pain and some of the key outcomes from this engagement. While I was preparing for the talk, I realized that inequities not only exist for Māori but also for other Indigenous populations and culturally and linguistically diverse (CALD) populations with persistent pain across the globe. In this blog post, I will share why inequities in pain management exist for Indigenous and CALD populations and what we can do to help address those inequities.
Silent suffering
“I used to be a queen bee,” said one of our Māori participants when asked about her persistent pain experience. “I don’t want to talk about it” was another statement I remember, as pain was perceived as a sign of weakness among Māori men. To fulfill multiple social roles, these individuals dealt with persistent pain with silent stoicism. In fact, most of the participants said this was the first time they were asked about their pain experience. What happens when they, our Maori participants, do seek help for pain management? From my interviews with kaimahi (community health workers) who often take whānau to their healthcare consultations, we found out that pain was mostly managed by pain medications and whānau did not receive explanations about their chronic pain condition. There were instances of racial bias, which negatively influenced further pursuit of pain management. In fact, referral rates to tertiary pain services are low for Māori and Pasifika (another CALD population in New Zealand).
Big picture
The disproportionate burden of persistent pain is a theme not only for Māori but also for other Indigenous and CALD populations living with persistent pain. What are some reasons for such inequities? One common theme is lack of representation of Indigenous and CALD populations in pain research, yet these are the very communities with high unmet needs. To briefly mention here a few plausible reasons (a blog post in and of itself!): a historical breach of trust by researchers working with those communities, excluding research participants because of a lack of English proficiency, and having pain questionnaires that are not culturally validated.
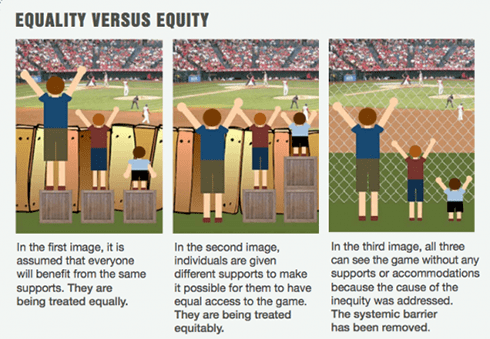
How can we address these systemic factors?
As a pain researcher, I am completely aware that I alone cannot solve the problem. However, change often starts within “you,” right? From my experience, I have strived to develop a meaningful partnership with our local Māori community, guided by our community partner and Māori researchers. This partnership was a result of mutual trust and respect that was built by working with communities to understand and address their needs. Similar examples of working with Indigenous and CALD communities living with persistent pain can be found here and here. As shown in the right-hand panel of the accompanying image, unless the causes of inequities (i.e., systemic barriers) are addressed, those inequities will remain. As a first step, we need to acknowledge and identify (from both a research perspective and a clinical perspective) the systemic barriers that exist and that contribute to inequities for Indigenous and CALD communities with persistent pain. As a pain scientist, clinician, or patient advocate, what is your “small step” to address inequities in pain management for Indigenous and CALD populations living with persistent pain?
Hemakumar Devan, PhD, postdoctoral fellow, University of Otago, New Zealand.
Patient Partners in Pain Research
Historically, clinical trials have involved clinicians and researchers creating the trial (i.e., making the decisions) and patients just participating in the trial (i.e., acting as the data source). More recently, there has been a move towards including patients as partners in the design and implementation of clinical trials. Given that the purpose of clinical trials is to improve patient outcomes, this approach makes sense. The first major government-supported initiative aimed at patient engagement with research was the INVOLVE program in the United Kingdom, which was launched in 1996. This program now gives patients training and encouragement to contribute to all levels of research, from design to dissemination. There are now similar initiatives throughout the world. Patient partner programs are now well established in some fields (such as cancer and AIDS) and there is increasing interest in the pain field in this area (for instance, see RELIEF related articles on IASP’s patient engagement initiative). But these programs have not yet been as widely implemented in pain research as they should be.
Pain is a highly subjective and personal experience that varies between individuals and pain challenges. Therefore, the lived experiences of pain patients and their families may help researchers to better understand how exactly pain impacts people’s lives, and this brings unique insight and ideas to the research process. Including patients in shaping the design and outcome measures of clinical trials improves the quality of the research. Patients can shape the direction of clinical trials by giving insight into specific concerns, priorities for research, and what questions researchers should be asking. These are parameters that researchers can only guess at. Patients can also give meaningful input on logistics, for example, whether both parents and children should answer questionnaires in trials that involve children. Patient partnership has also been shown to increase enrolment in studies; including patients in the decision-making process gives them a sense of ownership in the trial and they are therefore more likely to recruit others through word of mouth. Patients can also have a positive impact on the dissemination and reach of research by ensuring it is written up in accessible language and posting it in patient advocacy groups and websites (see related PRF podcast here). The patient-partner approach has already yielded positive results in the rheumatology field.
Patient partnership programs don’t just help improve research outcomes. By being given the opportunity to share their story and contribute to clinical and research decisions, patients are given a sense of empowerment. Patient involvement in setting research priorities will ultimately improve the quality of care and treatment of pain by ensuring that funds are allocated to projects that are likely to be useful and meaningful to patients. Being included in trials also gives patients a sense of purpose and personal fulfilment.
Although including patients in scientific projects is not always easy and may lead to extra workload and trials taking longer, the evidence suggests that the improved outcomes for all parties involved outweighs these potential negatives. Indeed, some have suggested that including patients in the decision-making process is often very rewarding and even fun!
Sherelle Casey, PhD student, University of Sydney, Australia.
Week 4: Monday, November 23, 2020
The Benefits of Clowning Around
Will a Humble Zebrafish Lead to a New Therapeutic?
What Does Expanding Access Mean?
“Despite all my rage, I’m still just a rat in a cage.”
The Odyssey of Changing Our Habits
How Does Capsaicin Both Cause and Relieve Pain?
A New Severity Classification System for Sickle Cell Disease
Part 2 – No Pain, No Gain: How Do Athletes Use Pain to Fuel Sporting Success?
The Good, Bad, and Confusing: The Role of Immune Cells in Spinal Cord Injury Pain
The Magical Influence of Words
The Benefits of Clowning Around
In 1986, Big Apple Circus Clown Care® was born. This organization, founded by Michael Christensen, a professional circus performer, sent clowns and other performers to pediatric hospitals around the United States. They became the first “clown doctors” and the practice of therapeutic clowning was born. It didn’t reach the public eye, however, until the movie Patch Adams was released. This movie is based on the life of Hunter “Patch” Adams, MD, who introduced clowning into his care as a young doctor in order to improve the experience of his patients. Therapeutic clowning is now a growing worldwide paramedical career. While most commonly seen in children’s hospitals, new programs are starting to bring this joyful practice to adult hospitals, geriatric care, and refugee or trauma situations.
Therapeutic clowning brings humor, games, and a positive atmosphere to patients and families. Wearing brightly colored medical jackets and red noses, and with pockets full of games and joyful tricks, therapeutic clowns help manage stress, loneliness, and fear in patients (see here and here). Positive effects have been seen on patients’ pre- and post-surgery anxiety levels, surgical pain, needle pain, and the stress and pain associated with treatments such as chemotherapy (see here and here). Not only do children appreciate the presence of clown doctors, so do parents, siblings, adult patients, and elders. Thankfully, few children endorse a fear of clowns (only 1.2% in this study!), meaning most pediatric patients stand to benefit from therapeutic clowning. Even staff members describe benefits of having clown doctors as part of their medical team, from increased productivity and decreased burnout to potential cost-saving outcomes.
There are multiple reasons why therapeutic clowning works! First, clown doctors provide an engaging and fun distraction from the medical procedure. They also offer a safe social connection in a scary, sterile environment. Clown doctors decrease stress and encourage laughter. This increases positive emotions, causing endorphins to be released and decreasing the body’s stress response. Together, these effects lead patients to experience more positive outcomes during medical care.
Clown doctors follow ethical guidelines to respect patient preferences, ensure confidentiality, and safeguard the patient’s experience. They face a challenging job of engaging with patients and improving their experience without hindering or impairing the work of the medical team. Thankfully, their presence is increasing across the world, bringing a sense of joy and positivity into medical situations that are often stressful and scary. In the words of P.T. Barnum, “The noblest art of all is to make others happy.” And I, for one, am incredibly happy that clown doctors and the practice of therapeutic clowning exist.
Mary Lynch, PhD, postdoctoral fellow, Indiana University School of Medicine, US.
Stories are powerful tools that “reveal things to us that we know but didn't know we knew,” as the philosopher Maurice Merleau-Ponty once said. I did not fully realize the impact of this idea until I recently completed a storytelling workshop organized for early-career scientists in New Zealand. We were a diverse group of scientists from all over the country doing research on topics like climate change, physics, and wildlife conservation. In this blog post, I share my transformative experience of how my story evolved, the power of listening to others’ stories, and how we might use stories for pain education.
The beginning
The six-session workshop started with a personal disappointment (you heard that right). I applied to the workshop thinking that I would learn how to facilitate one. Instead, the workshop focused on making scientists communicate their science via stories. But I couldn’t withdraw from the workshop because the participants were selected competitively from all over New Zealand. Therefore, I reluctantly decided to carry on. In the first few sessions, we discussed what a story is and what differentiates a story from a bunch of facts. If I understood it correctly, imagine a story as a thread that binds together a sequence of events – moments in your life. Depending on the length of the story (the thread), you will need more events (often emotional ones) to keep your audience engaged and connected to your story.
The middle
Even though I understood what makes a story, most of the participants, including me, were skeptical why we needed to know this. Then the workshop started to pick up the pace. We were asked to listen to podcasts of scientists sharing their stories. In the session, we discussed the "hook" at the beginning of a story, and dissected the emotional events and how those events were explained. Then came the most important part: we analyzed how those scientists integrated science in their story. Some scientists in the podcasts shared dramatic events (e.g., discovering a dinosaur egg) as part of their fossil research, and some shared quite personal experiences of how being a black person inspired them to study the psychology of fear. At this stage of the workshop, we were good story listeners, but everyone was thinking that we did not have such dramatic events or discoveries in our research. So what story do I have? Or who is going to listen to my story, even if I have one?
The end or a new beginning
In the last session, we had to come up with our own story and share it with our Zoom partner (really, there was no choice; we had to). With only 30 minutes before the workshop, I really felt like an imposter or a student who did not complete the homework; it felt like my school days. On my way to work that morning, I had been thinking about "What is my story?" and "Why am I doing pain research?" and realized that I had a personal experience of supporting my wife when she had a complicated pregnancy and intense back pain throughout her pregnancy. In the workshop, I shared how our initial joy of becoming pregnant turned into a painful experience because of her unrelenting back pain. In my story, I shared how, as a support person, I was unhelpful (in the first half of her pain journey) and how I transformed to support her in the second half of her pain journey. This is a reality for the whānau (family and significant others) of one in five New Zealanders who struggle to support their loved ones living with persistent pain, and so my passion for pain research was inspired by my real-life experience (I will tell you more in my podcast).
The moral of the story
From my first storytelling experience, I realized that we all have stories to tell, but it requires skilled facilitation (thank you Story Collider facilitators) and a safe space for all of us in order to share those stories and support each other. During the workshop, we all shared our personal stories, listened to each other and supported the construction of our stories. As a storyteller, it felt cathartic, and I also felt relieved and a sense of mastery for "discovering" a story of my own (which I did not have until a few minutes before the workshop). In a way, storytelling is therapeutic for the storyteller and for the listeners. To my readers and fellow pain scientists, what is your story? What inspired you to research pain? How can we use storytelling for people living with persistent pain as a therapeutic tool? What impact can personal stories of living with pain have on the storyteller and on others? Wish me luck for my first online story slam on 27 November! You can register here!
Hemakumar Devan, PhD, postdoctoral fellow, University of Otago, New Zealand.
Will a Humble Zebrafish Lead to a New Therapeutic?
Given that approximately 20% of adults in the United States suffer from chronic pain (with about a third of those experiencing high-impact chronic pain, which frequently limits life or work activities), developing effective ways to manage pain is critically important. Research into novel pharmaceutical approaches to pain treatment is one area that has garnered much scientific attention. While over-the-counter analgesics (NSAIDs, Tylenol, etc.) are fairly efficacious in treating minor acute pain, options for relieving severe acute pain and chronic pain are much more limited. Opioid-based analgesics provide great relief, but also pose numerous problems – they are prone to abuse, lose efficacy over time as people develop tolerance to them, and paradoxically, even have the potential to render patients more sensitive to pain following consistent use. Their highly addictive nature has contributed in large part to an “opioid epidemic,” with the US Centers for Disease Control and Prevention (CDC) estimating that 128 people die every day from an opioid overdose (from both prescription and illicit sources). Thus, there is an urgent need to develop different drugs to treat pain.
But how do we build better drugs?
There are numerous approaches to developing better analgesics. Some research groups are delving into the study of cannabinoids and other plant-based medicines that have been used as traditional remedies for millennia. Other groups are employing computational methods to design novel synthetic drugs that will target specific proteins involved in pain transduction. For example, computer-aided drug design is being enlisted to help in the engineering of molecules that will selectively activate endogenous opioid receptors in a specific/biased manner, bringing pain relief without eliciting adverse side effects. Once these designer drugs are made, they can then be tested for efficacy in animal models and clinical trials.
It’s also possible to do the opposite – an approach that our lab is taking.
Rather than try to engineer a specific designer drug based on how it might interact with a particular target and later examining its efficacy in pain relief, one project in our lab involves doing the reverse – screening a vast library of small molecules that bear some structural similarity to known drugs in a behavioral assay to see if any of these chemicals have the potential to decrease pain responses And we are able to do this because of a rather unlikely model organism: the small but mighty zebrafish.
Though fish might not be the first thing to pop into your mind when you think about pain research, larval zebrafish actually lend themselves quite marvelously to this kind of high-throughput behavioral screening. In our lab, we use a two-choice temperature assay in which zebrafish larvae are set on a plate, either side of which can be set to a specific temperature. Normally, zebrafish will strongly prefer the side that is set to their rearing temperature and will avoid the side that’s set at uncomfortably hot or cold temperatures. Exposing the larvae to noxious chemicals, such as chemical irritants, will amplify this preference, modeling thermal hyperalgesia. Administering an analgesic drug, however, can inhibit such temperature aversion, in both acute and sensitized conditions. Because zebrafish are prolific breeders, and because many individual larvae can be assayed at once, we have the ability to screen numerous candidate analgesics at a rate that would be impossible (or at least extremely impractical and expensive) with mice.
Through this drug screen, our lab has identified one potential novel analgesic, and we are currently in the process of determining its mode of action. A substantial portion of my postdoctoral work involves performing neuronal activity assays, imaging the brains of larval zebrafish to discover how this analgesic affects regional activity patterns when fish are exposed to painful stimuli. Once we have identified the effects of this molecule on neural circuitry in the zebrafish, the next goal is to characterize its physiological and behavioral effects in mouse models. If those experiments are successful, we hope that other, more translationally focused groups will take up the baton to investigate it further. Our lab is very much firmly in the realm of basic science, but it’s exciting to think about how, maybe one day, our humble zebrafish could have contributed to a new therapeutic.
Kali Esancy, PhD, postdoctoral fellow, University of Washington, US.
What Does Expanding Access Mean?
Do you ever open so many tabs in your web browser that you immediately become defensive about how important they each are when somebody points out that it seems excessive? Or maybe you are the person who minimizes your browser as someone approaches to escape the embarrassment altogether.
My tab count tends to skyrocket when I am reading about something interesting to me and I want to know more – and access to new information is just another tab away.
We are among those with the easiest access to information on the planet. But take a look here at an image that depicts the billions of people throughout the world who are still not connected to the Internet. There are an estimated 3.4 billion people globally who do not have Internet access. Really let that sink in for a minute.
The United Nations 2030 Agenda for Sustainable Development made a powerful declaration to combat inequality, stating: “As we embark on this great collective journey, we pledge that no one will be left behind. Recognizing that the dignity of the human person is fundamental, we wish to see the goals and targets met for all nations and peoples and for all segments of society. And we will endeavor to reach the furthest behind first.”
Access refers to so much more than having access to a web browser to accumulate tabs. Access refers to physical accessibility, financial affordability and social and cultural acceptability. After opening countless tabs to inform this post, I realized that I am guilty of talking about expanding access to pain information in a way that does not address all components of access equally.
Now that I have sufficiently depressed all of us, I want to share a story about expanding access to pain information that included multiple components of access. Last fall our team delivered a Comfort Ability workshop that included a parent and child dyad with visual impairments. In preparation for the workshop, we reached out to the Comfort Ability team at Boston Children's Hospital to inquire about their experience with access for those with visual impairments. Not only did they mail us manuals printed in Braille, they also spent an hour with us to thoughtfully tailor the in-person activities to be accessible for the family. It was a success story in minimizing barriers to access to a pain service.
Expanding access to pain information is clearly a common goal in the global pain community. And we are likely to continue to expand access in a myriad of ways. One of the most influential ways is to continue sharing information via social media, while taking care to ensure that the information is physically, financially, socially and culturally accessible for all, focusing on “reaching the furthest behind first.”
Wendy Gaultney, PhD, postdoctoral fellow, Oregon Health & Science University, US.
“Despite all my rage, I’m still just a rat in a cage.”
– Billy Corgan, The Smashing Pumpkins
Billy Corgan might have been referring to the fickle life of celebrity with the above, well-known line, but in light of this song being on repeat on my Spotify playlist, I started thinking about the debate surrounding lab rats. In this Chinese Year of the Rat (rats are associated with a plague again!), eLife published a really informative review article on the history of the Norway rat, a starting point for lab rats. As early as 1950, some scientists were questioning the utility of lab rats, because at this point these animals significantly differed from their wild ancestors. Although further studies suggested that an animal’s behavioral repertoire is not significantly changed by domestication, the utility of lab rats is still under scrutiny today because of a number of high-profile failures of translation from rodent to human.
In the pain world, one of these was the failure of promising preclinical data for neurokinin 1 receptor antagonists to translate to efficacy for pain in clinical trials. The reasons for this are probably multiple, but particular scrutiny has been given to relying on tests of allodynia and hyperalgesia as a read-out of “pain” in rodents when only 15%-50% of neuropathic pain patients actually have these symptoms. The practice of using exclusively male rats has also been discouraged, since women represent a majority of the chronic pain population. Furthermore, it has been shown that the etiology of chronic pain may differ between the sexes: males rely more on microglia and females more on T lymphocytes, revealing the need to investigate both sexes to develop analgesics relevant for women.
What has received less attention is the strain of rats or mice used. Different strains of both rats and mice seemingly have different vulnerabilities to developing allodynia after nerve injury. Patients with peripheral nerve injury or pathology do not always develop chronic pain, yet in preclinical research we often use rat strains that show a very high percentage of pain development. Fundamentally this seems to make sense because to study pain and reduce the waste of animals, it would be desirable for almost all rats in a study to develop chronic pain. Yet, are we missing something that relates to the human experience? An interesting study found that commonly used Sprague Dawley rats develop allodynia 85% of the time after spinal nerve ligation (SNL), whilst the closely related Holtzman rats only develop allodynia 50% of the time. Interestingly, when the authors injected formalin into the paw of these strains of rats, the second phase of the response, thought to be mediated by the action of inflammatory factors, was blunted in Holtzman rats in comparison to Sprague Dawley rats, and there were no significant subpopulations within strains. Electrophysiology showed that in a key brain area for descending modulation, the rostral ventral medulla (RVM), there were fewer pain “OFF” cell pauses and less pain “ON” cell bursts in Holtzman rats in the second phase of the response to formalin. This suggested that Holtzman rats may have a better descending inhibition pathway than Sprague Dawley rats.
Yet, if Holtzman rats have a higher-functioning descending inhibition pathway, why do 50% of them still develop neuropathic pain after SNL? Factors such as pre-surgery stress have been shown to prolong pain after paw incision, but it is unlikely that these Holtzman rats had different environmental experiences. An innovative study in Sprague Dawley rats showed that rats that had a deficit in fear extinction pre-SNL surgery went on to exhibit more aversive qualities of pain, including both audible and ultrasonic vocalizations in response to a noxious stimulus post-surgery, even though they showed the same amount of allodynia. This was correlated with greater activity in the central nucleus of the amygdala, suggesting “higher-order” brain areas may be involved in determining within-strain vulnerability to chronic pain.
So “despite all [the] rage” from failures of translation, rats still have a lot to offer pain research – but which strain?
Caroline Phelps, PhD, postdoctoral research associate, University of Arizona, US.
The Odyssey of Changing Our Habits
A warning to the reader: There is a little bit of statistics ahead. It sounds horribly boring, I know. I hope that you'll give me the benefit of the doubt and you allow me to …
… welcome you to a fierce fight in the statistical world! In one corner, the reigning champion: the so-called ANOVA, a widespread and heavily established statistical habit. ANOVAs are useful when you want to test whether more than two conditions or groups are different, for example, whether the mean height of people differs between Tokyo, Zurich and New York. ANOVA has a special right hook: the repeated measures ANOVA, which is used when the SAME group of people is compared in more than two conditions or at more than two timepoints. For example, whether the web page with the PRF correspondents’ blogs was accessed more or less often over the course of six weeks of blogging, compared to the beginning (hmmm, I'd like to know that…).
Now, this very right hook is also the weakness of the reigning champion. I will spare you the mathematical details (you can look them up in this short discussion), but let me tell you, there are some flaws. And one can do better … like the challenger in the other corner, the rising star: linear mixed models. Its approach to questions where the same group is observed over multiple conditions offers several advantages (again, the stats lovers out there can consult the link above). Its disadvantage: it's slightly more complex to understand … and it is NEW.
“Well … science shouldn't shy away from the complex and new, right? After all, research aims to explain unknown matters and sometimes those happen to be quite complicated,” thought my naïve, early-career researcher's brain once I discovered the strengths of linear mixed models. To my surprise: in 2015, the field of neuroscience used linear mixed models only about one out of seven times! Admittedly, the numbers are changing, and 2020 will probably show a different picture. But just recently, a friend of mine was asked during the review process by one of the reviewing experts: “Why did you not use a simple repeated measures ANOVA?" (instead of her linear mixed model analysis).
So, despite well-known advantages of the newer approach, there is obviously some hesitation to change old habits – to dethrone the ANOVA as the leading statistical approach for repeated measures analyses. Honestly, this discovery left me quite frustrated. It’s sad to mention that the ANOVA vs. linear mixed models fight is only one of many other examples where I have experienced a resilience to new, different approaches. I asked myself: shouldn't we dare trusting in new approaches where we have good reasons to believe in their value, although they have never been applied as such before?
I believe we should. I believe we should explore the possibilities of new ways, rather than desperately hold on to the old ways. Without a doubt, the old ways have proven themselves. But our world is changing. In order to keep pace, we should not be hesitant to modify our potentially outdated habits – even if it might resemble embarking on a long and tough odyssey.
Your critical "scienthlete,"
Laura
Laura Sirucek, PhD student, University of Zurich, Switzerland.
How Does Capsaicin Both Cause and Relieve Pain?
Capsaicin is a compound that is found in spicy foods like chili peppers and is the reason for the burn that we feel when we consume those foods. This occurs because capsaicin binds to a particular type of ion channel (TRPV1) that is usually activated in the presence of heat (43°C to 45°C). This means that binding of capsaicin is felt as heat. When capsaicin is extracted for use in creams and gels and then applied to the skin, this heat induces a pain response. The pain response is a protective mechanism to facilitate withdrawal of the area of contact, preventing a potential burn injury. Effectively, capsaicin applied on the skin tricks us into thinking we are being burned. It seems paradoxical, then, that capsaicin can be used both to induce pain in experimental models and as a treatment for pain.
Given that capsaicin makes us feel like we are being burned, it makes sense that it is commonly used as a method to induce experimental pain in both humans and animals. In rodent models, capsaicin is usually injected into the hindpaw, causing increased responses to both thermal and mechanical stimuli. These effects are accompanied by localized inflammation of the nerves in the skin that transmit nociceptive signals (see here for recent review).
In experiments using healthy volunteers, capsaicin cream applied to the skin is used to mimic chronic pain, as it leads to burning and aching pain and central sensitization (increased activity of pain receptors in the brain and spinal cord, leading to increased sensitivity to pain). Capsaicin can be used to assess both primary (at the application site) and secondary (in adjacent regions) hyperalgesia (defined as an excessive pain response to a normally mildly pain stimulus, e.g., a pinprick). Secondary hyperalgesia occurs due to central sensitization, mimicking many symptoms of clinical neuropathic pain. Topical capsaicin is a reliable model for testing of new analgesic compounds as it is an easily inducible, reversible model of central sensitization.
So how, then, can capsaicin also be a treatment option for neuropathic pain? Capsaicin (at low concentrations of up to 0.1%) is used in topical creams or ointments for relief of post-herpetic neuralgia (nerve pain caused by shingles), as well as arthritis and musculoskeletal pain. It turns out that when applied often, capsaicin has the opposite effect to the one-off administration protocols that are used to cause pain. That is, repeated applications of low dose capsaicin actually leads to desensitization (and a reduction in pain) rather than the sensitization (and increased pain) that occurs with single doses. This is thought to occur due to deactivation of certain ion channels (meaning that the nerve is unable to fire further nociceptive signals), as well as direct facilitation of withdrawal of nerve fibers in the skin (meaning fewer nerves are available to send nociceptive signals).
Unfortunately, these effects are not long-lasting and low-dose capsaicin treatments need to be applied four times a day to be effective. However, a single 30-60-minute treatment with a high-dose (8%) capsaicin patch can provide sustained pain relief for up to three months (but local anesthetic prior to treatment is needed as capsaicin can be very painful during application). Importantly, the magnitude of this pain relief is similar to gold-standard oral treatments such as gabapentin and is free of the problematic side effects (e.g., dizziness and somnolence) associated with oral treatments (see here for recent review).
So this is how one compound can have usefulness both for causing and relieving pain! Who would have thought that chili peppers could be so useful in the pain field?
Sherelle Casey, PhD student, University of Sydney, Australia.
The terms yin and yang are so familiar that they have become a part of the English lexicon. They originate in Chinese philosophy, where the concept of yin and yang describes how two seemingly contradictory forces are actually complementary and inseparable. Yin is the receptive principle, representing passive forces such as dark or cold, and yang is the active principle, representing opposing forces such as light or heat. This is a wildly oversimplified understanding of yin and yang; the principle has, in a way, served as part of the cultural DNA of China for over 3,000 years. However, for the sake of this blog post, the above definition will suffice.
The duality of yin and yang is the guiding principle of traditional Chinese medicine (TCM). It is believed that the relationship of yin and yang sustains the vital energy or life force of the body, qi, which circulates through invisible channels in the body, called meridians. In order for the body to stay healthy, it needs to maintain a balance of qi. When there is an obstruction in the meridian system, qi is unable to circulate throughout the body, resulting in a deficiency in yang, and it is thought that this is responsible for disease and illness. In order to clear the obstruction and re-establish the circulation of qi, needles are inserted into points of the body. This therapeutic technique is called acupuncture, a branch of TCM, and the points of the body the needles are inserted into are aptly named acupoints.
Acupuncture was formally introduced to the West in 1971 by the journalist James Reston in “Now, About My Operation in Peking,” an article for The New York Times describing his acupuncture therapy in China. Western critics often dismiss TCM as pseudoscience; indeed, there is no scientific evidence to support notions such as qi or meridians or acupoints, and it remains a controversial practice to this day.
Acupuncture has nonetheless been widely used for pain relief. Several clinical studies have shown that acupuncture therapy can improve chronic pain-related conditions, such as neck and shoulder pain or lower back pain, to name a few. In line with this, according to the US National Institutes of Health (NIH), “acupuncture appears to be a reasonable option for people with chronic pain to consider.”
We cannot make concrete conclusions about the effectiveness of acupuncture in controlling chronic pain. With that being said, in a comprehensive systematic review of randomized controlled trials between 2000 and 2018 investigating the effectiveness of acupuncture in comparison to different types of control interventions for chronic pain treatment, Chen and colleagues demonstrated good evidence that acupuncture offered better outcomes when compared to control groups that had received either no treatment or usual medical care.
While opioids and narcotics are still the go-to for pain relief, in light of the public health crisis that has been raging since the late 1990s (more commonly known as the opioid epidemic), should we not be putting more emphasis on exploring alternative, non-pharmacological forms of treatment for chronic pain management?
Isobel Parkes, PhD student, University College London, UK.
A New Severity Classification System for Sickle Cell Disease
Sickle cell disease (SCD) is a prevalent genetic disorder involving red blood cells and is associated with numerous health complications. The clinical course of the disease is influenced by a variety of factors such as age, psychosocial health and comorbidities. There are multiple different genotypes for SCD, however, there is a broad range of disease severity even within an individual genotype. This adds to the complexity of identifying categories of overall disease severity.
Currently, there is no universally accepted classification system of overall SCD severity. Thus, a recently published article by Nirmish Shah and colleagues reported on the development of a severity classification system for SCD by a multi-disciplinary panel of experts who reviewed patient histories and identified ratings based on multiple related disease factors (e.g., quality of life, risk of complications, etc.).
Upon reviewing patient histories, disease severity was broken down into a 3-level classification system, with a range of Class I (i.e., least severe) to Class III (i.e., most severe). Class I is defined as patients who have no end-organ damage or chronic pain and who have infrequent acute care visits from a vaso-occulsive crisis. Class III is defined as patients who have more than five acute care visits and/or who have severe damage to bone, heart, lung, brain, kidney or retina.
This classification system provides a preliminary categorization of disease severity, and the authors said that more studies further validating this system are planned. Although more research to further refine this system is needed, it provides an opportunity to make better predictions of health outcomes and disease course for patients with SCD. Predictions such as these would subsequently promote better patient care and improve quality of life.
Angela Pascale, PhD Student, Virginia Commonwealth University, US.
Part 2 – No Pain, No Gain: How Do Athletes Use Pain to Fuel Sporting Success?
(See Part 1 here.)
We see it all too often: Exhausted marathon runners limping their way through the finish line, fighting through unforgiving muscle cramps and aching muscle pain from lactic acid build-up, and with their lungs burning and gasping for air. Runners say that the pain usually starts at about the halfway point, where they contemplate dropping out of the race. But even with this agonizing pain (which could be amplified by extreme climates or previous injuries), athletes persevere to perform and, oddly enough, continue to return to the starting line for the next challenge. I remember numerous occasions when, as a competitive athlete myself, I would use this exertional pain to temporarily guide and push me to accomplish my athletic goals.
Reflecting now as a chronic pain patient, I wonder: How do athletes tolerate the pain experienced during sports? Even more interesting, how do athletes strategically use this pain to their advantage? For the most part, athletes have pain thresholds that are similar to everyone else’s, but what’s interesting is that athletes are willing to tolerate higher levels of pain for a longer period. Scientists have found that compared to the average person, athletes differ in their ability to deal with pain by using psychological and cognitive strategies.
Psychologically, athletes may perceive pain differently than nonathletes by framing pain as a challenge to overcome rather than something to be feared. Athletes may also tolerate pain better simply because they are more frequently exposed and accustomed to brief periods of intense pain during competition and training. This attitude towards, and previous experience or “practice” with pain gives athletes higher self-efficacy and greater confidence in their ability to control pain, compared to nonathletes. This difference between athletes and non-athletes suggests that pain tolerance can indeed be trained and learned over time.
Cognitively, athletes rely on two main strategies when experiencing exertional pain. One is dissociation, where the individual focuses on something positive to distract themselves and block out the pain (e.g., visualizing your “happy place”). Conversely, athletes also rely on association, where they actually focus on the pain (e.g., by monitoring bodily sensations or pacing oneself during a run). For athletes, how they use these techniques depends on the nature of their pain and the demands of their particular sport. From my personal athletic experience, dissociation works best for low- and moderate-intensity activities, such as jogging, while association is more effective during more demanding activities, such as weightlifting (especially when you’re trying to squeeze out that last rep of 12).
The unpleasant but common aspect of competitive sport is enduring pain. Yet, some of the most memorable sporting moments in human history involve athletes triumphing over pain. For most athletes, pain at times seems like the central reality of their existence but using various mental cues during competition can help them temporarily frame pain to their advantage.
Prab Ajrawat, MSc candidate, University of Toronto, Canada.
The Good, Bad, and Confusing: The Role of Immune Cells in Spinal Cord Injury Pain
A spinal cord injury can be devastating, with a wide range of complications presenting themselves in the weeks to months following the injury. A prevalent symptom, and one that can be very difficult to treat, is chronic pain. Patients can often be prescribed pain medications, such as opioids or pregabalin, that are used to treat a wide range of pain conditions.
Spinal cord injury researchers have long been on the hunt for a treatment that might be a little more specific or a little more effective than those currently offered. One area that looks promising is the interaction of the immune system with the injured spinal cord. After a spinal cord injury, the protective barrier surrounding the spinal cord is broken. This allows immune cells that wouldn't usually reside in the spinal cord for an extended period of time the chance to enter. Many of these cells might release compounds that will increase inflammation, such as TNF-α or IL-1β.
When a cell or compound seems to make a condition worse, a common approach in science is to get rid of it in a laboratory model and see how that changes things. Indeed, many companies now offer an array of mice lacking a particular immune cell or they provide many different pharmacological compounds that can remove this “bad” cell. The spinal cord injury research community has used these novel new mice or techniques to get to the root of the pain problem. Unfortunately, the problem doesn't seem as easy to solve as one might have first thought.
Take, for example, astrocytes. After a spinal cord injury, the astrocytes residing in the spinal cord will migrate toward the injury site, trying to contain the damage by blocking it off from the rest of the healthy cord, creating the “glial scar.” It was thought that this glial scar was maybe helpful at first but could be very detrimental in the long term. This blockade by astrocytes could be preventing the damaged neurons from re-growing and allowing patients to regain their normal functioning. But in mouse studies, when astrocytes were removed and the glial scar did not form, injured mice didn't recover any better, and the neurons didn't end up re-growing. In fact, the mice actually showed a worse recovery! It turns out that the role astrocytes played in containing the damage after injury was far more critical than we first thought.
Macrophages are another example. It had been known for some time that when macrophages first enter the spinal cord, they do a good job of digesting damaged tissue. However, an issue arises when they digest all the damaged tissue but continue to hang around and digest healthy neurons. But what happens when you prevent macrophages from entering the spinal cord? You guessed it: a poorer recovery! These infiltrating macrophages played an important role in reducing the activation of another cell type, microglia.
Although these studies don’t provide all the solutions to the problem of spinal cord injury pain, they have taught us some extremely valuable lessons about how immune cells interact with one another and with the nervous system, and about timing treatments so they have the maximum effect. In the end, it probably isn't just one immune cell or protein that causes spinal cord injury pain; it's perhaps about how these cells communicate and the overall environment of the spinal cord.
Courtney Bannerman, PhD student, Queen’s University, Canada.
The Magical Influence of Words
We are all aware of Dr. Martin Luther King Jr.’s famous “I have a dream” speech and the strong impact of this speech on humankind. In this speech, he begins each sentence with the words “I have a dream,” and this repetition, at the same part of successive sentences, is a form of speech called anaphora.
An “Aha!” moment happened to me when I learned that anaphora can be a potentially valuable method for clinical communication with our patients when we discuss the significance of active treatment options.
Last week I discussed some of the perceived challenges to exercise in people with chronic pain (see here). While reviewing these challenges, I realized that, to increase the uptake of exercise by our patients, primarily we need to change their beliefs (which are usually unhelpful) towards exercise, movement, and physical activity; beliefs influence our behavior and the actions we take. To achieve this objective, many psychologically informed physical therapies such as pain neuroscience education, cognitive functional therapy, acceptance and commitment therapy, and graded exposure and activity have been advised for patients with chronic pain in recent years.
Along with looking at the scientific literature, I have listened to some audio books (mostly 10-minute summaries) that can help us build the necessary skills to change the patient’s beliefs and communicate effectively, to help move the patient towards choosing active treatment options.
Motivating our patients to exercise can be carried out by asking the right questions and saying the right words. This idea is inspired by the book Exactly What to Say: The Magic Words for Influence and Impact, by author and sales trainer Phil Jones. He says, “The right words at the right time can make all the difference.” Getting a person to go from “no” to “yes” and to buy into our ideas happens in three stages, Jones says, and he suggests using a set of unique words at each stage. The three stages are Attention (Okay, I am listening stage), Interest (Okay, maybe stage), and Decision (Okay, I will do it stage). While Jones geared these ideas to sales and business situations, here’s how they can be adapted to our clinical settings to move patients towards exercise.
Attention stage (Okay, I am listening stage)
To capture someone’s attention, Jones recommends the questions below, which I have adapted to chronic pain management:
Question 1: “What do you know about” the cause of your pain and the benefits of exercise in pain?
If patients know something, they will be eager to learn if they are correct, and if they do not know anything, they would be eager to learn.
Question 2: “How open minded are you” to learn about the causes of your pain and the benefits of exercise?
Very few people wish to be perceived as closed minded, so naturally they would say yes and listen to what you have to tell them.
Interest stage (Okay, maybe stage)
In most circumstances, moving a person directly from no to yes is extremely difficult, so Jones describes an “Okay, maybe stage” in the middle. The specific words to use in this stage are “Just imagine” and the instruction can be:
Statement 1: “Just imagine” that you can go for a trek that you have been avoiding because of a fear of pain, and that you complete it with your family without provoking the pain.
Jones says that when a person hears the words “Just imagine,” the subconscious brain creates images of the scenario that we describe and creates interest in the options we propose.
Decision stage (Okay, I will do it stage)
Jones says that presenting a person with the following sequence of options can help make decision-making easier.
Statement 2: “The way I see it, you have three options”:
First you present the troublesome status quo (for instance, you say to the patient, “You can continue with your current approach, which doesn’t seem to be working for your pain”).
Second, you present a laborious alternative (“You can think about costlier and invasive treatment options for your pain”).
Lastly, you present the option you want the patient to choose (“You can choose to exercise for three to five days a week, which is simpler, more convenient, and more effective than the other two options").
Finally, we can end our persuasion session with an anaphoric phrase like King’s “I have a dream" speech. For instance: “Exercise reduces your pain, exercise improves your activities of daily living, exercise improves your self-efficacy,” and so on.
Y V Raghava Neelapala, PT, MPT, Assistant Professor, Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India.
Week 3: Tuesday, November 17, 2020
Pain Management in Primary Care
“No Pain, No Gain”: Consequences of the Normalization of Pain in Elite Female Gymnasts
“No Pain, No Gain”: Why Do Athletes Fight Through Pain? Part 1
Barriers to Physical Activity and Exercise in Chronic Pain
Art as a Tool for Science Outreach
The Eyes Are the Windows to the Soul
A Link From Hippocrates to Conditioned Pain Modulation
New Directions in Neuropathic Pain
As an Iowa farm girl, I grew up seeing the beauty and challenges of rural life. Weather is unpredictable and holds an unfair grip on income. A “trip to town” was planned when something was needed, and people only went to the doctor when there were no other options left. Many families in rural areas experience poverty, limited options for medical care, and isolation from common support services. Farm life is rife with accidents and injuries, and the manual labor involved often results in chronic pain. That being said, living in a rural area, especially in a farming community, brings many heartwarming joys. Rural communities are hardworking and tightknit. Families help each other, often stepping in and stepping up to help those in need.
Rural America is diverse. It includes Native tribal lands, ranches in the West, the Black Belt in the South, aging farmlands in the Midwest, and more. There are wide variations in race, income, institutional policies, and religious affiliations. Unfortunately, rural location and poverty increase the risk of having chronic pain. While there is some research on chronic pain in rural adults, the pediatric field is severely lacking. In order to really understand the pain experience of these children and adults, researchers will need to approach the rural community in a thoughtful way.
Often researchers are “outsiders,” not born and raised there like community members are. This can lead to distrust and suspicion, to feeling like the researcher doesn’t understand or have the rural community’s best interests at heart. Elders, town officials, and religious leaders could be approached to begin bridging the gap. Partnerships with groups like the National FFA Organization, 4-H, or Rural Assembly, and engagement with local schools, primary care offices, and co-ops can help researchers meet rural people. The tightknit feeling present in rural communities should be leveraged by having community members help develop the questions researchers ask and the methods they use. Given transportation barriers, inconsistent access to internet or other technology, financial constraints, and the lack of paid time off in rural life, it would be wise to use novel strategies to bring the research to the community. Once trust is established and thoughtful research has been conducted, researchers should maintain the new partnerships and give back to the community.
Rural individuals often live with chronic pain and their experience is understudied and undertreated. They deserve research that takes a deeper dive into the unique cultural aspects, resilience, and barriers they experience. If done well, by building trust and letting community members inform the research process, we could see great improvement in our understanding of and treatment plans for rural individuals with chronic pain.
Mary Lynch, PhD, postdoctoral fellow, Indiana University School of Medicine, US.
Have you seen the movie Joker? Growing up in India, movies are a great part of my culture. Although I moved to New Zealand a decade ago, we still try to catch up with all the latest Indian movies. Would you believe me that around 1,800 movies are released every year in India? It is a huge enterprise. Often people think about "Bollywood" as Indian movies, but there are 28 states with unique languages, and they all create their own movies reflecting the local culture and realities.
Before I get sidetracked, I wanted to share the impact of Joker and how movies represent people with long-term health conditions, including persistent pain, and how they influence societal health beliefs. I am one of the 100 million people worldwide who have seen Joker. My first take on watching the movie was how societal inequalities deterred the ongoing treatment received by the lead character, who was living with a long-term mental illness, and the detrimental consequences that followed. I was equally perplexed by which bits of the movie were real and which parts were illusions. Anyway, the movie perpetuates stigma and prejudice toward people living with mental illness, such that they were perceived as violent and harmful. This was the finding from a small-scale study conducted in New Zealand, which compared changes in prejudice levels in people who watched Terminator: Dark Fate or Joker
What does it mean for pain research? If watching a movie can reinforce public beliefs about, and attitudes toward people living with a long-term health condition, this could also apply to people living with persistent pain. Similar to mental health conditions, persistent pain is invisible, and associated social stigma devalues the realities of people living with it. I did a quick search using the search term “chronic pain” on IMDb, an online movie database, and found several movies listed. One of these movies is Cake, starring Jenifer Aniston. Although I have not watched the movie (I tried to watch it before I wrote this blog post, but life got in the way in the form of kids!), a previous study found that the movie represented the complexities of a lead character who was living with physical and emotional pain, dealing with loss and grief, and managing pain killers and addiction. A short film inspired from lived experiences I would also like to highlight Struggling to Be Me, a short film from the UK that synthesized research capturing the experiences of people living with persistent pain. This is another novel way of representing people’s journeys and struggles of living with pain, to help improve societal beliefs and address stigma. Showing this film to a group of pain clinicians inspired empathy and understanding of people’s day-to-day highs and lows of living with pain. Public comments in response to the film found that it gave those with pain a sense of validation and a powerful medium to explain to others what they go through every day. What’s next? Of course, I have to watch Cake this weekend. Beyond that, because of their potential reach, movies have a powerful role in addressing myths about health conditions. If done well, they can be used as a global pain education tool (see the IASP resource here). I am not saying that we should all start directing movies, but perhaps we can explore how public health messages are communicated via this art form and the impact those messages have on the public and on clinicians. Perhaps, in collaboration with lived experience experts (aka people with pain), co-creation of short films, using qualitative synthesis as a way of portraying the realities of living with persistent pain, could be one small step towards defeating social stigma and improving societal beliefs, providing support for living well with persistent pain. Hemakumar Devan, PhD, postdoctoral fellow, University of Otago, New Zealand. Our bodies are continually taking queues from our surroundings. When it’s hot, we sweat; when it’s cold, we shiver; and when it gets dark, we get sleepy (unless you are a very caffeinated graduate student). Our circadian rhythm is our body’s natural sleep-wake cycle. It’s why you might not be able to get those couple of extra hours of sleep on the weekend even when you don’t set your alarm. We take in signals from the environment that help set our circadian rhythm, mainly in the form of light. It’s one of the reasons it’s recommended to limit screen time before bed. Even though our body’s circadian rhythm is commonly called the sleep-wake cycle, it influences so much more than just that. Recent studies and reviews have demonstrated its importance in thirst, immune cell function, disease onset, and many more functions. A new, exciting line of emerging research is the circadian rhythm of pain. Research suggests that a patient’s pain is more intense at night and less intense in the morning, though it is important to note that not all patients experience a rhythm to their pain, and there do seem to be differences between men and women. This rhythm also seems to persist when patients are receiving pain medication. The reason behind this cycle of peaking pain could potentially be due to the circadian control of both the nervous system and the immune system. Many different inflammatory mediators that have been shown to be important in pain have fluctuating levels throughout the day, such as IFN‐γ and TNF‐α. For researchers who study pain, in both patients and laboratory models, this could signal the importance of making a note of when sensory testing or other sampling is being performed, and asking if the patient you are working with does night shift work. It is possible that these aspects could influence your results. In the age of personalized medicine, we will improve patient care as we discover more and more about circadian rhythms. This could be altering the doses of pain medication so that they would increase throughout the day. An altered dosing schedule could go a long way towards reducing tolerance and improving pain relief for patients. It is also possible that patients who do and who do not have a rhythm to their pain could benefit from more specialized and diverse treatment strategies. So, the next time you wake up at 7 a.m. on a Saturday because your body won’t let you sleep in, know that it’s your circadian rhythm edging you on to start the day. Courtney Bannerman, PhD student, Queen’s University, Canada. Pain Management in Primary Care If you have ever worked in a pediatric primary care clinic, or taken your own child to a busy practice, you will know that it can be a bustling place. Once checked in, families are escorted to exam rooms, where they wait for their provider to see them. Down the hall you can hear other doors opening, healthcare providers entering, doors closing. After what can feel like quite a long wait for caregivers and children, a provider walks in, apologizes for the delay, and proceeds to ask a list of questions about general health, behavior, developmental milestones, sometimes maternal health, and the list goes on and on. Pain is a common topic in the midst of the multitude of other topics that have to be addressed during these 15-minute visits (in the US) – from concerns about colic, to immunizations, to growing pains and minor scrapes, to abdominal pain that arises on Monday mornings before school. Managing pain in primary care is tough. There are about a hundred priorities to squeeze into a short conversation with a hungry child (or two) squirming on an exam table. So, before we can recommend best practices for pain care in any setting, we have to experience the real pressures within the context. And recognize the challenges that healthcare providers face. There are fantastic examples of settings in which pain management has changed substantially despite challenges. For example, NICUs today often look and feel different than they did 20 years ago. Pain management in NICUs not only became a higher priority, but language, training and practice shifted to integrate preventative pain care for preemies who require countless needle sticks. Currently, the demands on pediatricians and other healthcare providers in primary care settings are simply too great to dedicate sufficient time for pediatric pain evaluation and management. Those of us who specialize in pediatric pain management can and should actively advocate for pediatricians to have what they need to be able to address the ever-changing pain concerns through a child's life. And if you ask a pediatrician what they need more of to address pain, they will almost certainly answer, “time.” Wendy Gaultney, PhD, postdoctoral fellow, Oregon Health & Science University, US. “No Pain, No Gain”: Consequences of the Normalization of Pain in Elite Female Gymnasts I recently watched the Netflix documentary “Athlete A,” which follows the story of gymnast Maggie Nichols and the investigative journalists who exposed the sexual abuse of over 500 girls and young women (including nine former Olympians) by former USA Gymnastics team physician Larry Nassar. The film reveals the toxic culture of USA Gymnastics, with coaches physically and emotionally abusing the girls. One form of emotional abuse outlined in the film was gaslighting, with coaches telling girls that they were fine (or making it up) when they reported pain or injury. Since the release of the film, gymnasts in many other countries (including Australia, New Zealand, Britain, the Netherlands, and Belgium) have come forward with details of physical and emotional abuse at the hands of their coaches (see here, here, and here). Is it really any wonder, then, that so many former gymnasts retire from the sport in their late teens (usually due to injury), and often end up with lifelong chronic pain? As a former high-level gymnast myself, I can attest to the fact that pain is to be expected in gymnastics, with my coaches telling me to “embrace the pain” and “make pain your friend.” The “no pain, no gain” discourse promotes pain as necessary to improve performance. This is all well and good when applied to the “good pain” associated with conditioning training, but the normalization of pain encourages gymnasts to train through their injuries. Many gymnasts, particularly Olympians, will continue to train and compete through broken bones, stress fractures, torn muscles, and other severe injuries. An iconic example of this occurred in the 1996 Atlanta Olympics. Kerri Strug was the last to vault, needing a score of 9.493 to secure the gold medal for Team USA. On her first vault, she landed awkwardly, tearing two ligaments in her ankle and scoring 9.162. She was clearly limping as she walked back down the runway but, at the behest of her coach, performed the second vault and managed to stick the landing (scoring 9.712) before falling to the ground in agony, needing to be carried off (see video here). She was later carried to the podium for the medal ceremony. The decision to vault again ultimately ended her gymnastics career, with the ankle healing slowly and not well enough for a return to competitive gymnastics. It’s not just former Olympians who end up with lifelong injuries. A 2004 survey revealed an average of two injuries per retired gymnast, most commonly of the lower back, followed by feet, ankles, and knees. Aside from chronic pain, retired female gymnasts are prone to osteoarthritis, osteopenia, and stress fractures that occur well after retirement due to low bone density, muscle groups weakening (and no longer compensating for weak bones), and changed exercise patterns (see here). My ongoing knee issues (and the reason I required the knee surgery that triggered my CRPS) are at least partially due to my 15 years of gymnastics, and one of my teammates has recently undergone surgery to repair the ligaments in her ankle, which were lengthened due to rolling it one (or a few) too many times in her gymnastics career. After reading all of this you may be wondering why anyone would take up the sport of gymnastics. For some, it is the dream of Olympic success, for others it is parental pressure. Many go into gymnastics at a young age to help with development and co-ordination and end up falling in love with the sport. I was put into Kindy Gym as a two-year-old to help strengthen the muscles around my shoulder (as it would spontaneously dislocate a few times a month and not go back in for a few days), and despite explicit instructions that I was not to do high-level gymnastics (as I am hypermobile) I fell in love with it and didn’t want to stop. There is honestly no feeling quite like flipping and flying through the air or the sense of satisfaction that comes with mastery of a new skill. Simply put, most of us do gymnastics because we love it! Sherelle Casey, PhD student, University of Sydney, Australia. “No Pain, No Gain”: Why Do Athletes Fight Through Pain? Part 1 Picture this: The game is tied with three minutes left to play. The arena is packed with thousands of passionate fans chanting your name. The spotlight is on as you catch the basketball. You take a hard dribble to your left, easily bypassing the defender, and now have a clear path towards the basket. But just as you’re envisioning the glorious satisfaction of scoring, you suddenly hear a gunshot “pop” followed by excruciating pain, your leg giving out, and, as you look down, you see your Achilles tendon coiled up on the back of your lower leg. On April 12, 2013, this was the reality of the late (and great) Kobe Bryant. For many of us, an Achilles tendon rupture means an immediate trip to the local emergency department, but for athletes like Kobe, who continued to play for minutes, it’s a test of pain tolerance. There are many other examples of this, at different levels of sporting competition. In a 2012 published report from the British Journal of Sports Medicine, 39% of soccer players in the 2010 World Cup used painkillers before games. In youth, 42% of child athletes have concealed injuries to stay in the game. The question remains: Why do many athletes, at all levels of sport, consistently play through pain? Recent qualitative research examining rowers with lived experience of lower back pain found that a widespread culture of pain and injury concealment in sports may be to blame. Athletes internalize fears of isolation, neglect, and being perceived as “weak” when they are injured or experience pain. Given the “win at all costs” mantra and toughness culture in sports, many athletes only feel valued when they are physically well. Pain in sports has been normalized to the point where athletes are expected to “suck it up” and ignore their pain. This is further reinforced by the media, where commentators praise the “courageous” act of playing though injuries and high performance is prioritized over enjoyment and health. Unfortunately, these cultural norms lead many athletes to avoid openly disclosing their pain status so they may continue to compete. Whether they are professionals or amateurs, the lack of support and increasing pressure put on athletes forces them to return to play as fast as possible. Personally, I’ve seen many athletes push through their pain in order to maintain their job security, endorsements or scholarships, to accomplish their personal athletic goals, and to please fans, coaches, teammates, parents, or themselves. However, objectifying athletes as commodities who sacrifice their bodies for entertainment purposes negatively affects their relationship with their own health. Encouraging athletes to hide their pain can be a gateway to avoid seeking help and concealing other health issues, such as mental health problems and substance abuse. Having played basketball my entire life, I’ve seen more injured players in locker rooms and more painkillers in gym bags that I can count. I speculate that many players have not been 100% pain-free in a very long time. But when you think of sports as a career, aren’t professional sports teams the “employers” and athletes the “employees”? Don’t these sports teams have the responsibility to protect the welfare of their employees? Athletes have the right to report their pain and receive proper treatment without the fear of negative consequences or jeopardizing their career. The solution is not to push through pain, but rather to challenge the current sporting culture in order to create an environment where “sports ethics,” safe and evidence-based pain management practices, and the athlete’s welfare are prioritized. Stay tuned for Part 2 next week…. Prab Ajrawat, MSc candidate, University of Toronto, Canada. Barriers to Physical Activity and Exercise in Chronic Pain Happy Diwali (Indian festival of lights) to all the PRF readers! In last week’s post, I wrote about how patients with chronic pain tend to prefer passive treatments over active treatments. I shared that post with some of my students, one of whom shared the clinical experience of a patient with chronic gluteal pain who was prescribed strengthening and mobility exercises and, of all of them, foam rolling was the one that made the patient feel the best. So, I tried to search for research on barriers to exercise, and treatment preferences, in patients with chronic pain. I could not find many studies from India, but I will summarize some of the interesting findings reported in the existing literature and relate them to the Indian scenario, based on my clinical experience. I found that barriers to physical activity are generally described as physical, cognitive/psychological, financial, and cultural. Pain provocation Increased pain during activity and exercise is obviously the most frequent physical obstacle to participating in physical activity among chronic pain sufferers. Patients report that pain occurs after they start to exercise and they hesitate to complete the exercise session because of pain. Cognitive and psychological factors Fear of re-injury and pain is another factor that has been reported to limit exercise participation by people with chronic pain, which can be explained by the fear-avoidance model of pain. I have noticed that people with chronic pain acquire fear-avoidance beliefs from other patients (mostly from relatives and friends, listening to their painful experiences). Also, some health care professionals believe that patients with pain should avoid all physical activity and simply rest, and that is what they prescribe. A lack of knowledge and awareness about the benefits of exercise and physical activity is another barrier, as health literacy in India is limited and much less information in general is provided to patients about the significance of rehabilitation. Financial constraints Rehabilitation expenses are mostly out-of-pocket in India. One session of supervised exercise costs anywhere from 50 Indian rupees (INR) to 2,000 INR, depending on the location, whereas one analgesic pill (quick fix) costs approximately 5 INR and a hot pack costs 200 INR (which can be used for multiple sessions). So, in low- and middle-income countries, treatment choices are largely influenced by available finances and it seems easier on the pocketbook for patients to take an analgesic. Cultural aspects The influence of culture on treatment selection is something that I have learned while writing this blog. A scoping review by fellow PRF correspondent Hemakumar Devan and colleagues has offered relatable insights on the connection between cultural beliefs and treatment selection. In their review, the authors wrote that patients from some cultural backgrounds such as Iraqi, Iranian, South-Eastern European, Punjabi, Turkish and Moroccan backgrounds preferred passive treatments over active treatments. Importantly, in one study, UK Pakistani women said that exercise is “not a part of their culture” and that it is difficult to perform exercises at home. These results apply to the Indian context as well due to somewhat similar socio-cultural beliefs. The literature on barriers to physical activity and exercise is quite extensive and I have tried to summarize the most important ones. Knowing these barriers forms a framework in which to think of solutions to them, which I will discuss next week. Stay tuned… Y V Raghava Neelapala, PT MPT, Assistant Professor, Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India. Art as a Tool for Science Outreach When I hear the words “science outreach,” a few scenarios typically come to mind. There is, of course, the classic example of a scientist visiting a classroom (in person, or now, virtually due to COVID-19) to talk about their work. There are science summer camps and science nights and STEM fairs at schools, “Meet a Scientist” events and public lectures held at museums. There are endeavors like this PRF Correspondents program that help scientists become better writers for a variety of different audiences, and even social media platforms like Instagram and Twitter allow scientists to communicate their work across the vast expanse of the internet. But what about art? Can art also be used as a tool for outreach? Art itself often plays a role in science education, particularly in K-12 classrooms – who hasn’t had to draw a diagram of a butterfly life cycle, or make a shoebox diorama of an ecosystem? In the 7th grade, my science class teacher regularly made us take notes in picture format, which culminated at the end of the year in a massive scroll he dubbed “the never-ending story.” (To this day, most of what I know about the Kingdom Protista derives from drawing so many different types of algae.) My sister, in middle school, even helped decorate a eukaryotic cell cookie cake with candy and icing “organelles” as part of a science class project. Some of the activities the neuroscience community outreach group I was a part of during graduate school involved “neuron arts and crafts,” such as making neurons out of pipe cleaners and neuron face painting. Art is widely established as a teaching tool for making science concepts accessible and fun for children, but its potential for communicating science to adult audiences is perhaps not as explored as often as it should be. Science has long been a popular subject in art. From botanical illustrations to blown glass sculptures of invertebrates, from cross-stitches of nature scenes to shimmering prints of brain circuitry, people are often fascinated by the blending of science and art. The Society for Neuroscience’s annual conference even has an entire exhibit devoted to Art in Neuroscience, which this year was held virtually due to COVID-19. Science art is beautiful and interesting, to be sure, but in addition to that, I think it can be a valuable tool for communicating science and building bridges between scientists and non-scientists. A few years before I started grad school, other graduate students in my program at the University of Washington had similar ideas, and established Art Neureau, an annual neuroscience-themed art show hosted at a community arts center in Seattle. After I attended one of their exhibits as a first-year student, I enthusiastically joined as a volunteer on the organizing committee, for each of my remaining years in grad school. While participation was open to anyone – the only requirement was that any submitted work of art, whether it be 2D visual art, a musical performance, or an interactive exhibit, needed to pertain to neuroscience in some way – most of the people who contributed pieces were neuroscience-affiliated students, faculty, and staff at the University of Washington. Our show’s aims were multifold: to share neuroscience concepts with the public via art, to help foster conversation between scientists and community members, to highlight the similarities between art and science, and to show that scientists are complex, multifaceted people, with talents and interests beyond the lab bench. Like any event, organizing it could be a stressful and hectic experience, but the opening day of the show made everything worth it. Every single year was different, and every single year I was floored by what people came up with. The art we displayed wasn’t just pretty pictures of fluorescent cells taken on a confocal microscope; submissions included abstract paintings, mosaics, string art, glass etchings, music made from EEG recordings, Cajal-inspired cross-stitched neurons, photo collages, a hat knitted in the shape of a brain, ceramics, bags made from upcycled conference posters, balloon animal neurons, clothing, songs partially written by neural networks, and a steampunk machine that gave lessons on brain anatomy when you pushed certain buttons. Though I would hardly consider myself an artist, I had a great deal of fun every year making pieces that showcased my predilection for science puns, with titles like Cell Phones I: Cajal Me Maybe, Somewhere Over the Brainbow, and Mirror Neuron Neuron Mirror. Work contributed by community members was often deeply personal, shedding light on the way neuroscience impacted their lives. One year someone made a “memory box” for her father who was at risk of Alzheimer’s disease, and filled it with family mementos. Another year, someone contributed a series of self-portraits taken on their healing process from severe treatment-resistant depression, along with a hospital gown and cap they’d decorated with facts, statistics, and poetry about electroconvulsive therapy. I found these pieces especially to be deeply moving and powerful. Art Neureau didn’t just provide an enjoyable evening filled with interesting things to look at and listen to; it also provided conversations. I saw animated discussions over pieces that literally visualized a scientific concept, provided analogies in images, in ways that you might not encounter in a typical classroom/academic setting. I saw how these conversations not only served as educational moments, but also helped to humanize scientists. I saw scientists open up about their passions, both research and artistic. Conversely, I saw how art, especially art from community members, helps to humanize science – it is often easy for scientists, particularly those who aren’t also clinicians, to become absorbed in the minutiae of our cells and proteins and metabolic pathways – and this art gave a human face to what we study, grounded it in the implications science has for the very real lives of people. I saw how art had the potential to make science concepts accessible, and furthermore, to help break down the distinction between “educator” and “learner”; conversations over the neuroscience art felt less hierarchical, and more open, than conversations I’ve encountered at public lectures and similar outreach events. I’ve since wondered if the spirit of this is something that we, as scientists, should strive for in our other interactions with non-scientists, particularly given our current social and political climes, when fostering connections between scientists and the public is more important than ever. Though Art Neureau is an event that focuses on neuroscience and not explicitly on pain, I’ve thought recently about the potential art has as a communication tool in this context as well. Perhaps art can help illustrate scientific concepts and render them more accessible to patients, and maybe art can also be a vehicle for pain patients to express themselves in non-clinical terms. Perhaps art can play a role in helping researchers, doctors, and patients understand each other, and perhaps this understanding can be a tool to not only improve transmission of science and medical concepts, but also to forge trust, build relationships, and maybe even improve clinical outcomes. Kali Esancy, PhD, postdoctoral fellow, University of Washington, US. The Eyes Are the Windows to the Soul The popular proverb, “The eyes are the windows to the soul,” has been traced back to as early as Before the Common Era. The Roman philosopher Cicero (106-43 BCE) is quoted as saying, “Ut imago est animi voltus sic indices oculi,” which translates to “The face is a picture of the mind as the eyes are its interpreter.” A version of the proverb can also be found in the Bible: “The eye is the lamp of the body. If your eyes are healthy, your whole body will be full of light” (Matthew, 6:22-23). However, it has most commonly been attributed to William Shakespeare: “To thee I do commend my watchful soul/Ere I left fall the windows of mine eyes” (King Richard III, Act V, Sc.3, Line 117). Duchenne de Boulogne, a 19th century French physician at the Salpêtière, believed that it was the expressions of the whole face that were a gateway to the mind/soul, not just the eyes. He asserted that the human face was a kind of map, the features of which could be reliably coded into distinct categories of universal mental states, or emotions. Notably, Charles Darwin included the photographic slides from Duchenne’s monograph, Mécanisme de la physionomie humaine, in his fascinating work The Expression of the Emotions in Man and Animals, published in 1872. Here, Darwin famously claimed that nonhuman animals are also capable of expressing emotion through facial expressions. Interesting, you might be thinking (I hope you are thinking), but how exactly does this relate to the pain field? Mouse models are commonly used to study the pathophysiological mechanisms of pain. As we cannot directly measure pain in mice, indirect behavioural readouts, such as eliciting a withdrawal response of the paw, have long been used. The withdrawal reflex has offered an unprecedented view of stimulus-response relations in the somatosensory system, allowing the quantification of nociception and behaviourally relevant output. This, however, tells us very little about the unpleasant emotional experience and the higher order cognitive processes at play in pain. Using facial expressions to infer the emotions/internal brain state of the mouse (as Darwin posited over a century ago) could provide insight into the subjective pain experience of mice. Facial expressions have been used as an emotional readout in mouse models of chronic pain. Langford and colleagues developed the mouse grimace scale (MGS), where five facial features are scored: orbital tightening, whisker position, ear position, nose bulge, and cheek bulge; changes in these features are graded on a scale of 0 (normal), 1 (a moderate change), and 2 (severely changed). Despite the MGS being highly accurate, it is not all that sensitive. In order to elicit these changes in facial features, a considerable amount of nociception is necessary. In an elegant paper in Science, Dolensek and colleagues recently showed that mice exhibit stereotyped facial expressions that are specific to the underlying emotions, including a facial expression specific to the salient event of a painful tail shock. The authors took videos of the faces head-fixed mice made in response to an emotion event and used machine vision to extract features and quantify the differences between video frames. This revealed distinct classifications of facial expressions corresponding to specific emotion events, in which the valence or type of underlying emotion was not intuitively recognizable by a human observer. Machine learning could then predict the emotion captured in unlabelled frames of the face with more than 90% accuracy, the sophistication of the technique revealing features of emotion such as scalability in that emotion varies with stimulus strength, for example the intensity of the tail shock. The findings of Dolensek and colleagues offer researchers a vastly more sensitive technique to measure the emotional intensity of pain and infer the internal brain state of the mouse, without a doubt a significant advance on the use of grimace scales. Isobel Parkes, PhD student, University College London, UK. Disbelief, a hesitant laugh, a slight hint of irritation – this is what I saw in our students’ faces. And muffled murmuring, staring at the presentation slide in front of them. That morning, they had participated in an experiment: We had applied two different creams on two small skin patches of their arms, one on the left arm, one on the right arm. We told them (multiple times and with quite some enthusiasm) that one cream is a STRONG! analgesic that would reduce their pain sensitivity. The other cream, we told them, contained an ingredient that would sensitize their skin and would make heat stimuli more painful. We let the cream soak in, wiped the cream and measured their heat pain thresholds (the moment when you just start to perceive a heat stimulus as painful) on the respective skin patches. But here’s the thing: There were no different creams. The creams were 100% identical, merely some cheap lotion from the supermarket with no pharmacologically active ingredients. We had performed a very easy placebo experiment. Actually, we also performed a “nocebo” experiment because we suggested that one cream had a negative effect, in this case, a pain-increasing effect. This is the opposite of placebo, where expectations lead to a positive outcome, in our case pain reduction. So, I hope you are curious, what had happened to their heat pain thresholds? I'll reveal the secret: On the skin patch with the supposed “pain-killer” cream, the students had reported pain much later (at higher temperatures), compared to the skin patch with the supposed “sensitizing“ cream. Our experiment had worked! We were happy. But what about our students? I could tell that some of them felt tricked: “What? Me? No, I did not fall for a placebo … impossible.” One could say we were mean – after all, we had intentionally deceived them. But should we think about placebo as deception? Multiple times I have heard (or admittedly have even said myself) in a quite pejorative manner: “Yeah, but that's just placebo.” Or even: “It's placebo; it's not real.” Well, obviously it's very real! Pain perception does decrease. Importantly, it’s not only conscious measures of pain perception such as thresholds or pain ratings that show placebo effects, which could be biased, for example, if our students would have wanted to make their teachers happy. In fact, placebo effects are also observed in immune responses, blood pressure and so on. Two other fascinating aspects: Placebo can also work in chronic pain patients, and even if we know that we are receiving a placebo, as shown in so-called open-label placebo studies (see here). If we are in pain, does it really matter how we achieve relief? Is a “real” ingredient needed so that the relief is accepted as a true effect? Of course, for us researchers the exact mechanism of action matters. It mostly matters if we ask ourselves, can we develop treatment options that make use of the mechanisms underlying placebo effects? No matter how the placebo story ends, my opinion about placebo changed – now, I view it as evidence for the power of our mind and body rather than as deception. Thanks for reading, I hope to see you next week, Laura, your "scienthlete" Laura Sirucek, PhD student, University of Zurich, Switzerland. A Link From Hippocrates to Conditioned Pain Modulation Hippocrates suggested that “If a patient be subject to two pains arising in different parts of the body simultaneously, the stronger blunts the other.” Initially this does seem to fit in rather well with the ancient Greek form of medicine that included therapeutic techniques like bloodletting and cauterization, so why not think about providing analgesia by inducing a stronger pain? However, as odd as it seems, this “pain inhibits pain” phenomenon has wide-reaching applications in modern medicine. Known as conditioned pain modulation (CPM) in humans or diffuse noxious inhibitory control (DNIC) in animals, this phenomenon provides a way to dynamically measure descending inhibition in an individual. Typically, a painful “conditioning” stimulus is applied at a site on the body remote from the test stimulus. In the presence of a conditioning stimulus, higher pain thresholds for the test stimulus are often reported in healthy individuals. However, this is not always the case. CPM efficacy appears to exist on a spectrum in a population and this can have an effect on pain outcomes. One of the most enigmatic questions in pain research is why some people develop chronic pain and others don’t when they’ve had the same injury. An individual’s CPM ability could play a role in this. For instance, it has been shown that the worse an individual’s CPM, the more likely they are to develop persistent pain after thoracotomy surgery. Therefore, improving an individual’s CPM prior to surgery could reduce their likelihood of developing debilitating post-surgical pain. The utility of CPM doesn’t stop there. The degree of CPM is also predicative of response to different pharmacological therapies for neuropathic pain. In patients with diabetic neuropathy, those who had deficient CPM responded better to noradrenergic reuptake intake inhibitor drugs such as duloxetine, which enhance descending modulation, rather than to the other major option, the gabapentinoids, which decrease neuronal excitability. This is incredibly useful because it is often difficult to determine the best treatment for patients, so measuring CPM could get patients onto an efficacious treatment sooner. So how does this phenomenon work? The rodent equivalent of CPM is DNIC and it has been shown that the caudal-most region of the medulla, the subnucleus reticularis dorsalis (SRD), is necessary for the expression of DNIC in naïve rats. However, in a rat migraine model rat, a deficit in DNIC was alleviated by inhibition of the rostral ventral medulla (RVM), a region not thought to be involved in the response in naïve animals. Alongside the finding that blockade of 5-HT3-mediated descending facilitation alleviated loss of DNIC in a neuropathic rat model, this suggests that loss of DNIC in chronic pain models is due to greater descending facilitation from the RVM swamping the inhibitory response from the SRD. Further understanding of the CPM/DNIC response could aid development of new analgesics that specifically target this response to alleviate established chronic pain as well as prevent the incidence of persistent post-surgical pain. Therefore, the ideas of Hippocrates, the “father of medicine,” remain relevant and may inform future pain treatments. Caroline Phelps, PhD, postdoctoral research associate, University of Arizona, US. New Directions in Neuropathic Pain Neuropathic pain is pain caused by a lesion or disease of the somatosensory nervous system. It affects 7% to 10% of the general population and can be a significant burden for many patients. The burden of neuropathic pain is a result of the complexity of neuropathic symptoms, challenging treatment decisions and poor health outcomes. Neuropathic pain also impacts quality of life, due to increased visits to healthcare providers, increased drug prescriptions and morbidity from the pain itself. The burden and the impact on quality of life reveals the importance of future directions in treating and managing neuropathic pain. There recently has been progress in understanding the pathophysiology of neuropathic pain. That progress has led to new diagnostic procedures and personalized interventions and has highlighted the value of multidisciplinary approaches in managing neuropathic pain. The management of neuropathic pain primarily focuses on alleviating symptom; the cause of neuropathic pain is seldom treated. New directions for managing neuropathic pain have transitioned from the traditional approach where pain was managed by initiating treatment with strict pharmacological and complementary therapies before attempting intervention strategies. However, recently there has been an increased use of interventional therapies such as nerve blocks and neuromodulation. Additionally, psychological interventions that promote the management of pain and reduction of adverse effects are gaining more attention. Lastly, promising new directions for the management of neuropathic pain include phenotyping (i.e., analyzing observable characteristics of an individual resulting from the interaction of its genotype with the environment) and personalized pain medicine. By establishing phenotypic subgrouping of patients, more personalized pain therapy becomes possible. Personalized medical care entails that patients receive treatment based on their unique individual needs. This type of care considers, for example, the genetic makeup of an individual, and this is an encouraging new field of research. The field of neuropathic pain now has hopeful future directions for reducing the burden of pain and improving the quality of life for patients. Angela Pascale, PhD Student, Virginia Commonwealth University, US Week 2: Tuesday, November 10, 2020 When Raynaud’s Syndrome Hits the Lab Sweeping It Under the Rug: The Silent Pain of Endometriosis The Girl Who Cried Pain: A Gender Pain Gap? Chronic Pain and Opioid Addiction Which Came First, the Chicken or the Egg? Lost in Translation: The Gap Between Preclinical and Clinical Cannabis Trials Learning About Pain Through an Infant's Eyes Finding Sunshine and Support on Social Media Active and Passive Physiotherapy Treatments for Chronic Pain The Misconception About Palliative Care When Raynaud’s Syndrome Hits the Lab I love fall: the fiery foliage blazing against brilliant blue skies; the crisp, cold air; sweaters and comfort food; carving pumpkins; autumnal holidays and picking apples; the smell of woodsmoke as my neighbors light their first fires. My hands and feet, however, do not appreciate the transition into colder seasons. Though as a scientist I am hesitant to claim that I have a condition when I’ve never been formally diagnosed, I have all the textbook symptoms of Raynaud’s syndrome, a phenomenon in which the capillaries in one’s extremities overreact in response to cold or stress. For me, this starts to occur when temperatures dip below about 60°F, which honestly isn’t even that cold, and which the rest of my body doesn’t find that uncomfortable. Handling cold objects, and evaporative cooling – like what happens when I spray down my gloves with ethanol before working in our lab’s biosafety cabinet – will also elicit such “vasospasms.” This capillary constriction prevents blood from reaching my fingertips and toes, and will frequently make them turn a dramatic white, which would be a pretty neat parlor trick if it weren’t also accompanied by some rather unpleasant side effects. As most people have probably experienced, limiting the blood supply to a body part will cause it to go numb. There have been times that my fingers were so numb I had difficulty doing anything that requires fine motor skills, like texting or even unlocking our front door. Numb toes amplify my pre-existing clumsiness, and I have to be extra careful when navigating uneven sidewalks. However, I’ve also noticed something interesting: this numbness is frequently joined by pain. And on many chilly walks, the scientist in me has pondered the question: How is it possible to feel both numb and pain at the same time? How can my fingers be unable to sense mechanical stimulation, like the feel of my keys and a doorknob, yet somehow feel like they’re on fire at the same time? How can the cold (or rather, my overreaction to it) blunt one sense, and amplify another? Does the answer lie in the vasoconstriction? It has been well documented that applying limiting blood flow to an area (e.g., via ischemic compression using a blood pressure cuff) will block the conduction of A-fibers, the broad class of peripheral sensory neurons that includes those responsible for detecting touch, well before C-fibers, the group that includes neurons responsible for detecting temperature, itch, and noxious stimuli. Very well, my scientist brain said – that explains the numbness to tactile stimuli while still being able to experience other sensations. But what about the pain? Overreactive blood vessels can explain the numbness, but I’ve experienced pain when exposed to innocuous cool temperatures that ordinarily wouldn’t activate any cold-sensing nociceptors. I initially hypothesized that this hypersensitivity might have something to do with gate control. The gate control theory, first proposed by Melzack and Wall, postulated that A-fibers had the capacity to block C-fiber signaling via inhibitory interneurons in the spinal cord. It explains why we might feel relief when we rub a painful injury or scratch an insect bite. While the concept of gate control has been challenged, expanded, and refined over the years, the ability of mechanosensory neurons to inhibit nociceptors has been demonstrated. In my Raynaud’s-afflicted fingers, could the ischemic block of my A-fibers remove the “brake” on my C-fibers, allowing them to send volley after volley of unrestricted signals to my brain, which interprets this increased transmission as pain? Or is the mechanism entirely different? It could also be plausible, my scientist brain argued, that my experiences are similar to people with ischemic pain, where hypoxic conditions due to limited blood supply lead to the upregulation of inflammatory molecules and subsequent overexpression of certain receptor proteins on nociceptors. The impression I got from a brief foray into the literature, however, was that ischemic pain frequently is a more chronic condition, unlike the acute burning I experience, so I’m also hesitant to put all my eggs in that explanatory basket. What is the final answer? I don’t know. A few cursory literature searches haven’t yielded any satisfactory conclusions about Raynaud’s-associated pain, so I’m mostly left with my hypotheses and curiosity, and a hope that maybe one day other researchers will find out. As for me, though, I’ll still enjoy fall – I’ll just have to be extra vigilant about bringing along a good pair of mittens or a hot beverage on outdoor excursions! Kali Esancy, PhD, postdoctoral fellow, University of Washington, US. Sweeping It Under the Rug: The Silent Pain of Endometriosis My normally energetic and chatty girlfriend (unless she’s hungry) sat there silently in the car, with her arms wrapped around her knees and a look of excruciating pain on her face. Whenever I asked her to describe the pain she feels from endometriosis, it’s always simultaneous stabbing pain in her uterus and lower back, reminiscent of the popular Robaxin commercial. My girlfriend is just one of the 190 million women worldwide who experience endometriosis. It is one of the most common gynecological conditions, in which endometrial tissue grows outside the uterus and encapsulates surrounding reproductive organs, resulting in scar tissue and adhesions. This causes severe pain; for some women it can resolve after a couple of days, while for others it lingers with no end in sight. From my girlfriend’s experience, I’ve learned that extreme pain from endometriosis is an endurance sport, with minimal treatments and no cure. Endometriosis is poorly understood, rarely researched, often misdiagnosed, and from her experience, hardly mentioned during the patient-physician visit. Digging deeper, I’ve come to learn that a lack of awareness, unclear understanding of what “normal” menstrual pain is, and the social stigma attached to openly discussing reproductive health are common hurdles for seeking treatment. However, pain is entirely personal and, importantly, self-reported, and THAT matters. Many healthcare professionals often think menstrual pain is natural and just something women deal with. However, severe menstrual pain should not be dismissed as normal but rather talked about openly and approached with a clear, focused pain management strategy. Many women have to convince their doctor that their pain is real, and one of the most frustrating aspects of living with endometriosis, arguably, is the delay in getting it diagnosed, with an average delay of six to 10 years. Having earlier conversations about the long- and short-term effects of endometriosis, such as potential infertility, pain during sex, fatigue, gastrointestinal distress, and chronic joint pain is also critical to inform patients. As pain researchers, we are failing to provide evidence-based information on endometriosis and potential therapies because of a lack of research funding towards female-specific pain conditions. This can be attributed to the “gender pain gap” where female pain conditions have historically been sidelined. This is a neglected area of research that, oddly, uses male animal models (comparing apples and oranges?). Given this institutional gender discrimination and bias among the research and medical community, there have been minimal improvements in the diagnosis and treatment of endometriosis over the past few decades. We owe it to ourselves to invest in women’s pain research in order to expand our knowledge of endometriosis and develop new ways to treat and even prevent the condition in the future. Prab Ajrawat, MSc candidate, University of Toronto, Canada. "The Girl Who Cried Pain": A Gender Pain Gap? I recently read a review in Frontiers in Cellular Neuroscience describing current progress in elucidating the mechanisms underlying chronic pain in endometriosis. It left me feeling exasperated. How is it that we still know so little about the associated pain of a debilitating condition that affects one in 10 women? But I, and I am sure you, reader, have an idea of what the answer to this question might be –an answer that lies within a much larger conversation about gender discrimination in the diagnosis and treatment of pain. The notion of gender bias with regard to how seriously women’s experiences of pain are taken is not novel and has been discussed at length. Numerous studies have attested to the prevalence of sexism in the treatment of pain, with a seminal study being Hoffman and Tarzian’s 2001 literature review, “The Girl Who Cried Pain: A Bias Against Women in the Treatment of Pain.” The legitimacy of women’s pain has often been denied. This is in part due to the historical belief that women are just not credible sources of their own lived experiences (remind me, who/what defines a credible source?). Pain management has been particularly susceptible to this belief because of the subjectivity inherent in reporting one’s own pain and the lack of objective measures. These historical attitudes and beliefs endure. They continue to shape contemporary pain management on subconscious levels. A rather fine example of this is that, on average, it takes eight years to be diagnosed with endometriosis, where the primary symptom of chronic pain is regularly dismissed as menstruation pain. The normalization of pain surrounding menstruation is another issue altogether. To add to the already rather bleak note (apologies), this perennial dismissal of women’s pain has translated to a lack of research funding to study pain pathologies predominately experienced by women, including period pain and endometriosis. So how do we be proactive in addressing these gender-based disparities? Hoffman and Tarzian’s literature review was published almost two decades ago and yet still rings woefully true today. Daniel S. Goldberg, a faculty member in the Centre for Bioethics and Humanities at the University of Colorado Anschutz Medical Campus, argues that because discrimination in pain is embedded in the foundation of our social structures, if we really want to resolve this problem, we need interventions on a deeper level than interpersonal strategies like anti-stigma training. Is it time that the pain field seriously reckoned with how the patriarchy and structural inequalities profoundly influence pain management? Isobel Parkes, PhD student, University College London, UK. Chronic Pain and Opioid Addiction OxyContin, a sustained-release formulation of oxycodone, is a brand name almost synonymous with the prescription opioid epidemic in the United States. Recently, the manufacturer, Purdue Pharma, reached an $8.3 billion settlement deal with the US Department of Justice for the role its aggressive marketing had in fueling this epidemic. In a manner eerily reminiscent of when heroin was introduced as a medicine that was supposedly less addictive than morphine, Purdue repeatedly advertised a less than 1% chance of addiction with OxyContin for treatment of pain disorders. Yet this conclusion was based on studies of acute pain, as opposed to long-term opioid treatment, in their target patient group with chronic non-malignant pain. It is difficult to discern what constitutes addiction in chronic pain. A 2015 systematic review estimated the prevalence of problematic opioid use to be between 1% and 81%. The reason for this massive range is perhaps due to a lack of understanding of what constitutes problematic abuse in chronic pain conditions. While in opioid seeking for recreational use, dependence and addiction are present together, chronic pain patients may be dependent on opioids for pain relief without what is seen as problematic addictive behavior (see here). In fact, a line of thinking has been that chronic pain patients may demonstrate pseudo-addiction, where their repeated requests for more opioids is due to insufficient opioid pain relief as opposed to an addiction to euphoric effects. This has led to justification, as well as an opening up of the market, for the treatment of non-malignant chronic pain with opioids such as OxyContin. Yet chronic pain patients may be particularly vulnerable to addiction. Chronic pain and addiction share similar neurocircuitry, suggesting one may lead to another. For example, a decrease in activity in the medial prefrontal cortex (mPFC) has been observed in chronic pain patients. The PFC plays an important role in top-down cognitive control and dysfunction of the mPFC is associated with inflexible habitual behavior, perseverance and behavioral disinhibition, all key symptoms of addiction. Rodent studies may be key in understanding the predisposition of people with chronic pain to addiction. For instance, the infralimbic cortex (equivalent to the human ventromedial PFC) has been shown to be essential for extinguishing drug-seeking behavior in rats. Interestingly, in an arthritis rodent model, researchers reported that infralimbic cortex inhibition of the amygdala was impaired. Fear extinction behavior has also been shown to be impaired in a rodent model of pain, suggesting that extinction mechanisms may be impaired in chronic pain, leaving patients vulnerable to ongoing dependence on opioids. However, while there are some interesting parallels to be drawn between chronic pain and addiction neurocircuitry, a lot remains to be understood about the vulnerability of chronic pain patients to addiction. Increasing our understanding of this could potentially protect future generations from prescription opioid addiction epidemics. Caroline Phelps, PhD, postdoctoral research associate, University of Arizona, US. Which Came First, the Chicken or the Egg? We’ve all heard the saying, “You’re never really alone.” It’s very true, but not in the way you would first think. Our body is teeming with trillions of microorganisms, such as bacteria and viruses. Together, these microorganisms comprise our microbiota. Our most extensive microbiota population –and one that has been receiving a lot of attention recently – is located in our intestine. The gastrointestinal microbiome refers specifically to the collection of microbial genetic material residing in your gut. Over the last five to 10 years, this has been a fascinating new frontier of research. Changes in the microbiome, commonly referred to as gut dysbiosis, have been shown to occur in people who experience digestive disorders and those who live with various other conditions such as cardiovascular disease, depression, or autism spectrum disorder. The pain research community has also keyed into this potential goldmine of possibilities. In recent years we have seen the emergence of many papers showing a role for the microbiome in chemotherapy-induced pain, fibromyalgia, and many other conditions characterized by the presence of pain. In fact, my PhD project seeks to understand the role that the gut microbiome may play in spinal cord injury pain. I commonly face the question, “Does the spinal cord injury itself cause gut dysbiosis, or is it the treatment of the injury that causes gut dysbiosis?” In some cases, it could also be that the changing microbiome could cause or contribute to the onset of certain illnesses. These questions are applicable to almost all of the different conditions in which the microbiome has been shown to play a role. Take, for example, depression. Because of the gut-brain axis, which is the communication taking place between your brain and gut, and the amount of neurotransmitter produced by the gut microbiota, it is possible that dysbiosis could result in the onset of, or worsen, depressive symptoms. It is also likely that people living with depression could experience an altered lifestyle or diet, or be taking medication, which could affect the gut microbiome. There is also a great deal of work that needs to be done to better understand if and how the changing microbiome affects someone’s overall health. Changes in microbial populations do not necessarily mean that a patient’s broader health outcomes are affected. Indeed, many research papers show differences in microbial communities between people who live with a health condition and people who are healthy. It is important to note that the microbiome can be altered with diet changes, if you own a pet, if you exercise, and by many other things, but this doesn’t mean that some of these people are unhealthy. At the end of the day, research looking into the gut microbiome holds a lot of promise, but there is still loads of work to be done. However, it is very tempting to imagine the day when a patient’s pain and quality of life could be drastically improved through antibiotics or probiotics. So give your body’s microbiota a little extra appreciation. They are always working hard to keep you healthy! Courtney Bannerman, PhD student, Queen’s University, Canada. Lost in Translation: The Gap Between Preclinical and Clinical Cannabis Trials The Cannabis sativa plant and its constituents (phytocannabinoids) have been used for millennia to treat a range of medical conditions, including (but not limited to) pain and migraine. Recently, medicinal cannabis has received a lot of interest as an alternative treatment option for chronic pain. While it is portrayed as a silver bullet by the media, clinicians have far more diverse opinions. I’ll never forget my first conference presentation, where my question time turned into a lively debate among a few clinicians who were staunchly “anti-cannabis” and a few who were equally staunchly “pro-cannabis.” It is interesting that preclinical studies overwhelmingly suggest that cannabis is a highly effective treatment for neuropathic pain, while clinical studies are generally far more equivocal (despite a large body of anecdotal evidence). Indeed, recent meta-analyses suggest that cannabinoids have only modest efficacy against chronic pain. In this week’s blog, I’d like to discuss some of the possible reasons for this gap. Reason 1: Different cannabinoids Of the 400 compounds contained in the Cannabis plant, just two have been well studied: D9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is the primary psychoactive constituent, while CBD is the primary non-psychoactive constituent. Clinical studies have focused on whole leaf marijuana, isolated phytocannabinoids (THC, CBD, or combinations thereof), and synthetic THC analogues. In contrast, preclinical studies have largely examined synthetic cannabinoid agonists, with far fewer examining the effects of THC/CBD. Reason 2: Different delivery routes In preclinical studies, cannabinoids are generally administered via an injection (subcutaneous or intraperitoneal). But in clinical studies, cannabinoids are delivered by inhalation (smoked or vaporized), orally, or via the oromucosal route (as sprays). This is an important distinction since, unlike injectable routes, the oral route is subject to significant first-pass hepatic metabolism. Reason 3: Different pain measures Most preclinical studies use allodynia and hyperalgesia as effect measures, utilizing techniques like von Frey hairs and heat/cold to induce pain. These only affect around half of human neuropathic pain sufferers, with spontaneous pain being far more common. Recently, there has been a move towards evaluating spontaneous pain in rodents (grimace scale and gait, weight-bearing, and behavioral analyses), so watch this space! Reason 4: Different populations Preclinical studies use a highly homogenous population (genetically and in terms of the underlying pathology). In contrast, human trials are very heterogeneous and include a range of pain conditions, often not distinguishing between disease types (e.g., chronic non-neuropathic vs. neuropathic pain). Additionally, there is still a trend in basic science to use male animals only, whereas the majority of human chronic pain sufferers are female. There are many more reasons why findings from preclinical studies have not translated to clinical trials, but these are a few of the main ones. Do you agree? What is your opinion on using cannabinoids to treat neuropathic pain? Sherelle Casey, PhD student, University of Sydney, Australia. Last week, I received an invitation email asking to give a talk on “co-design” for a departmental seminar. Well, very rarely do people invite early-career researchers to give a talk, and with the ambition of adding two extra lines to my CV, I said "YES" within two minutes of that invitation email. I would have emailed within a minute, but that would have looked quite desperate! Now starts the tough part: I actually have to prepare for the talk! Having been involved in three co-design projects, I am reasonably confident I can pull together a talk. However, I do not want to talk about individual projects; rather, I want to focus on my reflections upon doing co-design projects with people with persistent pain, including Māori community members. Here are some of my key reflections as a fledgling co-design researcher. Confusion with terminology I am fascinated that there are so many terms used interchangeably to describe public and patient engagement in health research. People often use patient engagement, patients as partners, patient partner involvement, co-design, co-creation, co-production, participatory action. At the core of patient engagement is involving end-users in the design, delivery, and implementation of health research. This varied terminology means you cannot adequately search for relevant studies in scientific databases. As an experiment, I tried the aforementioned terms combined with “health research” in the Google Scholar database; each search gave me different results. In other words, if we do not use the same language, it is difficult to efficiently search and synthesize these studies to improve our understanding of this evolving methodology. Is it effective versus is it the right thing to do? Patient engagement is an evolving way of doing health research, and not everyone is on board (looks like a common theme in 2020). People who are critical of this approach ask, If you involve patients in your research, how it will improve my research? Show me the evidence for improved outcomes for treatments that were co-developed with patients compared to treatments that were not. On the other hand, if treatments are designed to improve outcomes for patients, why not have their say in designing those treatments. From an ethical, moral, and social perspective, it does make complete sense (at least to me!). There is emerging evidence to support patient engagement; if done meaningfully, the treatments have improved ownership and uptake with communities (see a New Zealand example here). What is meaningful patient engagement? The final conundrum in this evolving method is how researchers and patients can work together in a meaningful way. There is a risk that patient engagement becomes tokenistic, and a power imbalance between researchers and patients may deter meaningful engagement. From my experience, there are two potential reasons for this. First, funding systems are not prepared for this method, at least in New Zealand. This is because, in a funding application, you have to justify clearly why you think this research is needed (background) and here is how to achieve it (methods), laying out how many participants will be needed in a trial. As meaningful engagement takes time, there will need to be allowance for leeway in pre-defining your primary outcome and analysis plans. The second point, when I started to look for examples of meaningful engagement or co-design (this was a couple of years ago), I really struggled to find actual examples of papers from researchers explaining "how" they engaged and the impact of that engagement for researchers, for patients, and on their overall research outcomes. There is emerging guidance on how to report patient engagement, but there need to be more reports of co-design teams sharing their experiences, which will be a huge learning opportunity for researchers, patients, and funders alike. It is not doom and gloom There are emerging initiatives in the pain field to achieve meaningful patient engagement. The GAPPA initiative from IASP sets precedent by working with a global coalition of pain patients and researchers. I had the pleasure to facilitate a pain education webinar on behalf of the NZ Pain Society, which included a recorded session from Joletta Belton (co-chair of GAPPA) along with four lived experience experts titled “Nothing about us without us” – a coalition of lived experience experts in Aotearoa (the Māori name for New Zealand; check out the recording here). Personally, working with lived experience experts made me more human and humble, resulting in research that is mutually empowering and meaningful. I will finish off with this quote from one of our lived experience experts whom I am working with: “To know that pain researchers are willing to walk their talk, to listen carefully, and act compassionately in order to create better outcomes for pain patients is both a welcome relief and an encouraging step in the right direction.” Hemakumar Devan, PhD, postdoctoral fellow, University of Otago, New Zealand. Learning About Pain Through an Infant's Eyes My niece recently turned one year old. Most of her life has been confined to the walls of her home and the straps of her stroller as she is walked through her neighborhood. The COVID-19 pandemic has significantly restricted her physical social network beyond her parents during her first year of life. My sister-in-law's smiling but tired face is the one that provides the majority of social interaction and comfort. Now of course, this intense bonding and exhaustion is not a novel experience for new parents…. (ENTER global pandemic, stage left.) The global pandemic substantially changed social interactions. Many of us have learned, through textbooks and experience, of the critical importance of social interactions for child development and learning. We also know in the pediatric pain world that pain is also socialized to a large extent during childhood. A large proportion of learning happens with parents and caregivers; however, other social relationships likely offer opportunities for learning about pain as well. I am not sure about your family Zoom calls, but ours do not tend to detail our daily aches, pains, and recent minor injuries. We know that children learn how to respond to their own pain from watching others experience pain, and from how others react when they are in pain. At this point in time, it is not unrealistic to assume that my niece has only seen her own parents experience pain. And, further, her own pain experiences have been in the presence of her parents, almost exclusively. Which brings me to ponder the question: How might restricted social networks from the global pandemic affect children's access to other social interactions around pain? And how might cumulatively reduced opportunities to learn from others' pain experiences affect children's pain over time? I can tell you that, in the one Zoom call in which I did witness my niece bump her head while playing, she immediately reached to her father for comfort, he calmly checked her head for injury, kissed her single tear, and shifted her attention to the toy she was reaching for. In that moment, I felt quite confident that she will turn out just fine. Wendy Gaultney, PhD, postdoctoral fellow, Oregon Health & Science University, US. Finding Sunshine and Support on Social Media Living with chronic pain can be an isolating and lonely experience, especially for children and teens. While up to a third of kids experience recurrent or chronic pain (see here and here), my pediatric patients often tell me they don’t know anyone else with chronic pain! With our current pandemic-related restrictions, we all have to be creative in how we find our social connection. Social media is one way for individuals with lived experience and professionals alike to connect with others all across the world who understand. Who is using social media to talk about their pain? About 50% of all teens talk about their health online, ranging from feeling sick, to mental health concerns, or to having a chronic illness. Girls and women post about their pain and health on social media more than their male counterparts. Among pain conditions, fibromyalgia and headaches or migraines are most frequently talked about online. Why do they use social media? There are many reasons for posting to social media, including seeking connection and sharing experiences. Writing about chronic pain can help individuals create a story or narrative (see here and here) and give meaning to their experience. Pain is an invisible disability, so posting stories and pictures increases visibility for chronic pain. Feelings of loneliness and isolation go down as users see a face that they can connect with. Often people turn to social media to express their feelings and obtain emotional support. Retweets, comments, likes, and reactions can act as “cyberhugs,” giving peer recognition and support. Feeling seen, heard, and understood can truly be sunshine on a cloudy day for everyone, especially people with chronic pain. How can scientists and clinicians leverage social media to help more individuals with chronic pain? Engaging with individuals with lived experience on social media is a complicated but important outreach avenue to consider. Diagnosis-specific questions and treatment recommendations are often posted to social media. Approximately half of youth with chronic pain use social media to find health information. Parents, caregivers, and adults with chronic pain also seek support and recommendations for pain management online. So … it’s time for experts to get on board! Use social media to increase visibility of chronic pain, communicate support for those with lived experience, and share evidence-based treatments. Be mindful to share research findings and general care recommendations in a way that is engaging and easy to understand. This can be done through creating infographics, avoiding jargon, and highlighting the patient experience. While confidentiality and other safety concerns are legitimate issues to address, engaging as a professional on social media is important. We can bridge the unfortunate gap between publishing research findings and actually improving the lives of those who live with chronic pain. Mary Lynch, PhD, postdoctoral fellow, Indiana University School of Medicine, US. Active and Passive Physiotherapy Treatments for Chronic Pain “Sir, research indicates that active treatments should be encouraged in the management of chronic pain, and passive treatments do not appear to have much effectiveness. But our patients tend to prefer or mostly improve with passive treatments. What should we do in such a situation? Should we continue delivering these passive treatments or should we discard them completely?” Every year my postgraduate students ask me this question multiple times, and honestly, I do have a very tough time answering this question. I myself asked this question to a very senior pain researcher when I started my teaching career six years ago, and he said, “It’s up to the individual patient-therapist to decide.” I also observe at least one argument a week on this issue over social media, our favorite resource for knowledge these days. It is very well recognized that physiotherapy is an essential component of the multidisciplinary rehabilitation of chronic pain. However, what constitutes an effective physiotherapy program for the management of chronic pain has been a matter of academic and clinical debate. Broadly, physiotherapy management options for chronic pain are categorized as active or passive. Electrophysical agents, manual therapy techniques, dry needling, and taping are some examples of passive treatments, while active treatment options include exercise, physical activity, graded activity, and graded exposure programs. Five, 10, or 15 sessions of passive treatments initially (mostly as a package), followed by a few sessions of supervised or home exercise is the modus operandi for most (if not all) physiotherapy clinics in India. The package may include a combination of any number of passive treatment options, depending on the availability at the clinic and the skills of the therapist. What are the reasons for this bias towards passive treatments? First, there is the belief of patients and health care professionals that pain is caused by tissue damage and that injured tissue needs to be protected/rested until it is healed. Any form of movement or activity may worsen the tissue damage and worsen the pain, and hence should be avoided. Second, patients and health care professionals prefer quick fixes and short-term gains over long-term improvements. Most patients say that they feel better after a session using an electrical modality or a manual technique such as myofascial release than after a session of exercise. When the pain relapses after a few days, they would want these same passive treatments that have worked earlier. There can be many other reasons. But the important question here is how we can put the evidence into practice. I wonder how physios in other parts of the world addresses this issue! Here is one way of answering this question: Instead of viewing it as a matter of active versus passive physiotherapy treatments, we can consider it as active AND passive treatments, and try to deliver the best of both worlds to our patients. No one form of therapy has been shown to have large superior effects when compared to others. Evidence-based practice involves integration of best available evidence, clinical experience, and patients’ preference. So our modus operandi should not be biased toward one of type of therapy (active or passive), and it is rare in physiotherapy practice to deliver only one type of treatment. In other words, we can begin with a few passive treatment sessions to ease baseline pain and swiftly progress to active treatments when the patient is ready. Maybe it’s more relevant to identify the right time to make the switch from passive to active therapies and ask what skills are required to do that. Y V Raghava Neelapala, PT, MPT, Assistant Professor, Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India. The Misconception About Palliative Care November is National Hospice and Palliative Care Awareness month. Palliative care and what it means, however, remain elusive concepts. The misconception about palliative care is the belief that it is simply end-of-life care and support for a patient with a terminal illness. There is, however, much more involved in the meaning of palliative care. Palliative care is defined as an interdisciplinary approach that provides comprehensive psychosocial, physical, and spiritual care focusing on both prevention and relief for patients and families facing chronic and/or life-limiting illnesses. Thus, palliative care does not simply mean end-of-life care, but instead is a specialized approach of treating and alleviating symptoms of patients with serious or life-threatening illnesses. It is about improving quality of life. A contributing factor to this misconception of palliative care is the inaccurate belief that it’s the same concept as hospice. However, palliative care is different from hospice in two main ways. One is that the patient can continue to receive both curative and aggressive kinds of medical treatment for their illness while receiving palliative care. Second, in order to receive palliative care, patients do not need a provider to certify they have a terminal illness with a life expectancy of six months or less. One of the most crucial goals in palliative care is pain relief. Many serious or life-threatening illnesses such as cancer, liver disease, or congestive heart failure are conditions where pain is a common symptom. Palliative care services thus include an interdisciplinary team with expertise in pain management. Research has indicated that these teams improve pain control in patients. Hence, raising awareness of palliative care could improve pain management, and subsequently reduce suffering and improve quality of life for patients over the course of their illness. Angela Pascale, PhD Student, Virginia Commonwealth University, US Imagine you get the following task: Put your hand in ice-cold water for 30 seconds and tell me how painful it is. Sounds fairly easy, right? Well, this is psychophysics, a very frequently used tool in pain research. By definition, psychophysics assesses conscious experiences (e.g., pain) resulting from a physical stimulus (e.g., cold water). And here comes my first story about a surprising experience: Psychophysics is far from easy! It's like opening Pandora's box: Once you peer through the lid, you are bombarded with all the pitfalls and traps of psychophysics and their nasty consequences. Two aspects struck me as particularly sneaky: rating scales and instructions. Getting back to your hand in the cold water, this means: How should you quantify the pain you feel and how do I explain the task to you? I guess most people know some kind of rating scale. The most common rating scale in pain research goes from 0 ("no pain") to 10 … and here comes the first pitfall. 10 for what? I give you two options: "Most intense pain imaginable" or "Most intense pain tolerable." Which one do you pick? It might make a difference. Because the worst pain you can imagine (maybe something like giving birth) might not be the pain you are willing to tolerate during an experiment for a study (hell, no!). I realized that there is no right or wrong with regard to the anchor you set – but I also realized that the choice is trickier than you think. And that pain rating scales always deserve a second thought. I became similarly cautious with instructions. Verbal communication, such as instructions, is powerful. I honestly forgot how closely people can listen! (Maybe this is because physics classes taught me how easy it is NOT to listen). And how much they can remember! It somehow reminds me of telling a story to a child – the details matter (and don't you dare change them throughout the story). They also matter in psychophysics; I learned that even single words can have an unwanted influence or create confusion. I also learned that instructions should be carefully chosen – and never underestimated. In sum, be careful when opening the box of psychophysics. As comfort: Pandora's legend says that hope will remain in the box 🙂 See you next week. Your "scienthlete," Laura Laura Sirucek, PhD student, University of Zurich, Switzerland. Week 1: Monday, November 2, 2020 My Path to Studying Affect and Cognition in the Experience of Pain A Confession From a “Scienthlete” A Famous Philosopher With a Lot to Answer For Itchy Zebrafish and Now a Look at the Brain The Mystery of Spinal Cord Injury Pain A Biopsychosocial Approach to Improving Pain Outcomes Engagement and Resilience in Pediatric Pain A Binocular View of Pain: From Patient to Researcher When One Door Slams Shut, Break Another One Down Let's Talk Prevention – of Chronic Pain Kia ora koutou (Greetings, everyone) from New Zealand. My passion for investigating persistent pain started when I studied the gate control theory of pain during the second year of my physiotherapy degree in India. It gave me the beginnings of an understanding of how we experience pain and helped me get extra marks on my exam when I cited it to explain how physio treatments work (thank you, Melzack and Wall)! Fast forward a few years to when I was a new graduate physio and managing people with phantom limb pain, which made me realize how complex changes in the brain can lead to unrelenting pain. I tried my best to explain why someone would experience pain when they no longer had an arm or a leg. I ended up leading a small-scale clinical research study comparing mirror box therapy versus mental imagery for managing phantom limb pain. This was a clear tipping point when I realized my interest and research focus was on how treatments work and why, and for whom. A decade ago, I moved to the University of Otago, School of Physiotherapy, for postgraduate studies, where I continued my exploration of pain research in people with amputation. My PhD explored the connections between amputation, asymmetrical trunk movements, and low back pain. What started as a biomechanical exploration ended up embracing the complexity of psychological and social aspects of pain management. I am so glad I did a qualitative study during my PhD asking the patients, "What do you think is causing your pain?" I always thought that I would research pain mechanisms and management in people with amputation for the rest of my career. Wait – here is a plot twist: I moved to Wellington (the capital city of New Zealand) for my postdoc, where I started to work on a small research project with a tertiary pain service. I sat through one of their group-based pain management programs for 12 weeks. This experience significantly deepened my understanding of patients’ persistent pain journeys, their invisible suffering, and how they shared and cared for each other during the course of the program. Their discussions and questions about pain neurophysiology were equivalent to 100 books! During my postdoc, I am exploring how we can support self-management for people living with persistent pain and their whānau (family and significant others) using technology (e.g., apps, websites, and online-delivered treatments). These technologies, when co-created with people living with pain and culturally tailored, can potentially enhance access to and address inequities in pain management, particularly for Indigenous and culturally and linguistically diverse communities. I am a big fan of researching with people who have lived experience of pain and valuing their expertise, and I am passionate about changing societal beliefs and attitudes towards people living with pain and their whānau. In this age of misinformation and selective reporting, I strongly believe there is a clear case to be made for science communication. If done well, it can save lives. It certainly did in New Zealand (inspired by our NZ prime minister, of course). I would like to bring a similar logic to communicating about pain by ensuring that cutting-edge evidence and knowledge on pain science is translated into a language or medium tailored to those communities with highly unmet needs. I am really looking forward to being a PRF Virtual Correspondent over the next couple of months. I will strive my best to write about the contemporary (and controversial) topics of pain management, focusing on equity, culture, and pain. Hemakumar Devan, PhD, postdoctoral fellow, University of Otago, New Zealand. My Path to Studying Affect and Cognition in the Experience of Pain In George Orwell’s book 1984, the protagonist says, “Of pain you could wish only one thing: that it should stop. Nothing in the world was so bad as physical pain.” As the daughter of a chronic pain sufferer, I became aware early on in my life of the dearth of therapies that can make the pain stop. As a result, when I started my undergraduate degree in physiology, I began voraciously reading about pain. After finding Ronald Melzack’s Puzzle of Pain at a second-hand book shop for 50p, I considered it my bible for understanding the basics of pain. His interesting case studies inspired me to think of pain as an enigmatic experience as opposed to just a sensation, and his lucid explanations of the basics of pain really helped me when I started my pain research career. During my PhD, I was fortunate to have three supervisors: one focusing on depression and the other two on pain. My studies were primarily informed by the latest work on negative affective states and cognitive alterations in depression. I really relished the opportunity of this different angle of looking at the pain experience and the novel behavioral techniques I could employ in a rodent model of chronic pain. This work infected me with the enthusiasm to further study affect and cognition in the pain experience. Despite my being a homebody, always happier sitting at home in the UK with a good book, a cup of tea, and a biscuit, my enthusiasm for my research led me to pursue a postdoc in the United States with Frank Porreca at the University of Arizona. I had always admired his work on the aversive nature of pain. Despite a rocky start of homesickness triggered by the alien landscape of the desert, I embraced my new research project. Initially, I got to work on the role of the canonical stress response and heightened kappa opioid signaling in the amygdala in descending modulatory control of pain as well as the aversive nature of pain. Recently, I have been carving my own niche in the lab, investigating how cognition is impaired in chronic pain and how this impairment may actually drive the maintenance of pain. So far, we have found impairments in cognitive flexibility (the ability to adapt behavior to changing outcomes) in a rodent model of chronic pain, with the suggestion that the animals appeared to use a different, perhaps habitual, learning strategy to overcome their impairment in cognition. I have also been investigating short- and long-term memory impairments in chronic pain, varying task difficulty, and assessing the effects of analgesics to better understand the nature of the deficit. I’m excited to be part of the PRF Virtual Correspondents Program. I have always enjoyed science communication, whether it takes the form of talks or presenting posters to fellow scientists, teaching undergraduates or chatting to the general public when volunteering on stalls at science festivals. However, this year, with COVID-19 restrictions I have felt lost, so I’m looking forward to this PRF course! Caroline Phelps, PhD, postdoctoral research associate, University of Arizona, US. A Confession From a “Scienthlete” Let's begin with my confession: I had never planned to do a PhD. When I started my studies in biology – out of pure interest rather than having a career plan in mind – my dad used to joke: “One day, you'll be Dr. Laura Sirucek” (Dr. referring to a PhD). My reaction was always the same: laughing and “sicher nid!” (the Swiss expression for “absolutely, definitely NOT”). Anyways, I was too busy playing volleyball, practicing twice a day and fighting for my pro athlete career. What was it that changed my mind? Maybe it was the surprise of meeting numerous athletes doing a PhD (leading to a revision of my assumption, “Aren't all PhD students boring and nerdy?”). But thinking about it more closely … is that surprising? Not really. Doing a PhD has numerous parallels to being an athlete, which I discovered step by step – daily challenges, the will to pursue a goal, frustration tolerance (sound familiar?!), teamwork, determination and, above all, passion. Most likely it was the latter that paved my way to a PhD: a newly discovered passion for pain research, which awoke during my master’s thesis on sensory assessments of pain perception and grew steadily towards the end. I couldn't let go of my fascination with mechanisms behind central sensitization and its suggested psychophysical assessments such as temporal summation of pain, conditioned pain modulation, etc. (already nerdy myself, I know!). Now, I am following up on this passion in the second year of my PhD in neuroscience at the University of Zurich, in the Department of Chiropractic Medicine at Balgrist University Hospital, under the supervision of Petra Schweinhardt. My project aims to integrate psychophysical and brain imaging outcomes to advance our understanding of central sensitization in people with chronic low back pain. Dear PRF and RELIEF community: I am truly honored to participate in the PRF Virtual Correspondents Program. This is an exceptional opportunity to learn, to create, to share. What I would like to share with you during this journey are stories – stories about gripping scientific experiences I’ve had. Maybe you will find them fascinating, too. And maybe – if I did a good job as a storyteller – you will tell your friend, who tells a friend … and together, we will make science communication happen. Sincerely, your nerdy “scienthlete,” Laura Laura Sirucek, PhD student, University of Zurich, Switzerland. A Famous Philosopher With a Lot to Answer For There is a long-standing disagreement among many in the pain field over the relative importance of central mechanisms versus peripheral mechanisms of pain. Quite simply, Descartes and mind-body dualism have a lot to answer for. Central or peripheral pain is not a clear dichotomy, and more sophisticated models appreciate that pain is an experience where cognitive and sensory processes are intricately linked. One cannot think of either central or peripheral pain in isolation if progress is to be made in elucidating the pain experience and finding effective therapies for persistent pain. This is where my PhD research (hopefully…!) comes into play. I am aiming to explore the interplay of bottom-up flow of information from noxious input to attentional modulation and top-down descending modulation of pain. I am a first-year student in the MRC doctoral training program at University College London, and I am interested in understanding pain as a model of learning and action. Pain, at its core, is motivational – to coordinate both short- and long-term behavior in order to minimize harm. My research specifically focuses on how immediate protective behaviors, such as the withdrawal reflex, are embedded in the wider pain circuitry, and how these protective behaviors are influenced by context and attention. These questions I am asking fall within the realm of basic research, and the translational relevance of these questions is sometimes hard to articulate in public discourse. Discovery science is, as a consequence of this, notably underfunded. Science communication is crucial in engaging scientists and non-scientists alike to positively contribute to the conversation and advocate for scientific research. I am therefore, firstly, very much looking forward to beginning my science communication journey here as a PRF Correspondent, and secondly, as a very early-career scientist, who is very new to the field of pain research, I’m thrilled to have this platform to cultivate my scientific curiosity and share current research with you over the next couple of months that I am particularly excited by right now. Isobel Parkes, PhD student, University College London, UK. Itchy Zebrafish and Now a Look at the Brain Hello, PRF world! Kali here, corresponding from Seattle! I am a fairly recent graduate of the neuroscience program here at the University of Washington – I was lucky enough to defend my PhD about a week before COVID-19 shut everything down – and have stayed on in my dissertation lab as a postdoctoral fellow. My work in Ajay Dhaka’s lab in graduate school largely focused on the question of whether fish can experience itch, and if so, identifying the neurological mechanisms underlying this important sense. I will gladly talk anyone’s ear off about itchy zebrafish if they’re interested, but to make a long story short, our lab identified a novel mechanism by which the nociceptive receptor TRPA1 can be directly activated on different populations of peripheral somatosensory neurons to encode distinct sensations of itch and pain, and discovered that this rudimentary mechanism was also present in mice. As a postdoc, I’m finishing up experiments from my graduate research aiming to identify factors that distinguish highly sensitive, itch-tuned TRPA1 neurons from the less-sensitive pain-encoding TRPA1 neurons. I’ve also hopped onto a newer project in the lab that’s examining higher-order pain processing, focusing on the neural circuits that underlie aversion to painful/unpleasant stimuli and how certain prospective analgesic drugs might affect the functioning of such circuitry. As someone who spent her entire graduate career looking mostly at peripheral somatosensory neurons, I've certainly had an enlightening experience probing the brain instead! Science outreach, in the numerous ways it can manifest itself, is incredibly important to me. Science does not exist in a vacuum. In order for people to understand, appreciate, trust, and support science, scientists need to clearly communicate their work and its importance in ways that are accessible to communities outside their own circles. Effectively relaying science to the general public as a whole is critical to inspiring the next generation of scientists, and ensuring that they reflect the diversity of our nation’s populace, securing financial support for the sciences, and protecting both public and environmental health. Effective communication is one critical tool for establishing (and in many cases, rebuilding) trust between scientists and the general public. Such a sense of trust is all the more important in our current society, given the prevalence of climate change denialism, anti-vaccination sentiment, and even resistance to public health measures that could limit the transmission of COVID-19. I want to be the best scientist I can be, and I believe that means not just locking myself in the Ivory Tower (or, more realistically, basement laboratory) of academia, but also taking an active role as an educator and communicator. I’m excited to have this opportunity as a PRF Virtual Correspondent to hone my science communication skills, as well as learn a lot from my fellow Correspondents! Stay tuned for future posts about our lab’s pain-related research, musings about science communication, and more. Maybe I’ll even tackle some topics outside of my research wheelhouse! Kali Esancy, PhD, postdoctoral fellow, University of Washington, US. The Mystery of Spinal Cord Injury Pain I am a fourth-year PhD student at Queen’s University in Kingston, Ontario. I started on my journey to understand spinal cord injury pain – mainly how the immune system influences pain outcomes – during my undergraduate thesis. After completing my bachelor’s degree in biochemistry, I began my doctorate in the laboratory of Dr. Nader Ghasemlou. Although I originally started my project because of an interest in the biochemical mechanisms underlying disease and injury, I discovered a passion for studying pain. I currently investigate the microbiome’s role in influencing the immune system after a spinal cord injury, and how it can affect pain and locomotor outcomes. A substantial amount of evidence shows that the microbiome dramatically changes after a spinal cord injury, in both patients and laboratory animals. We also know that mice and rats with more pronounced gut microbiome changes show more severe locomotor deficits, anxiety, and depression. We don’t fully understand how these changes alter the immune or pain response after injury. Through my work, I hope to better understand the connections between these systems. My lab uses behavioral and locomotor assessments and follows up on our findings using molecular techniques. Although much of my research is working in the wet lab and with rodent models, I try to stay grounded by engaging with patient partners and listening to patient testimonials. I’m very excited to begin my term as a PRF Virtual Correspondent! I believe recent events have shown us the importance of effective science communication. The public is interested in receiving accurate scientific information, and researchers are moving towards a more interdisciplinary approach to their work. I am incredibly grateful to PRF for this opportunity to interact with people passionate about pain research and treatment to share ideas and information. Courtney Bannerman, PhD student, Queen’s University, Canada. A Biopsychosocial Approach to Improving Pain Outcomes Hello, PRF readers! My name is Angela Pascale, and I am currently a health psychology PhD student at Virginia Commonwealth University. I am very excited to be part of the PRF Virtual Correspondents Program! Throughout my life, my personal, educational, and professional experiences have fueled my research interests in investigating psychosocial factors that influence pain, particularly pediatric sickle cell disease (SCD)-related pain. My goal as a pain researcher is to implement pain reduction, management, and prevention interventions within a pediatric SCD population. I became interested in pain through my experience working at an inpatient palliative care unit. It was through this experience where I noticed factors such as mood and family support influencing pain outcomes across different chronic diseases. Further, an observation that was exceedingly apparent was how pain was ubiquitous for all patients in the unit as well as how pain negatively impacted quality of life. Thus, I became interested in understanding ways in which biological, psychological, and social factors influence pain, both individually and by interacting. Similarly, my passion for SCD-related pain began through working with patients with SCD in the palliative care unit, and continues to grow through the guidance and expertise of my graduate advisor, Dr. Cecelia Valrie. Through my pain research thus far, I have realized how crucial scientific communication is within this field. It is crucial to engage a wide variety of audiences, such as healthcare professionals and members of the public, when relaying research findings. Additionally, it is of particular importance to have scientific communication skills when assessing and implementing pain reduction, management, and prevention interventions. I am truly looking forward to improving my scientific communication skills and being able to implement them throughout my pain research career. Angela Pascale, PhD Student, Virginia Commonwealth University, US. Engagement and Resilience in Pediatric Pain I was drawn to pediatric psychology before I knew it was a possible career. Volunteering with the Children’s Miracle Network in college taught me the wide-reaching and often long-lasting effects that chronic pain and other chronic health conditions have on the lives of children and their families. Early engagement with families during their medical journey through clinical work, research partnerships, and advocacy has the potential to positively shift our patients’ trajectories. Currently, I am a postdoctoral fellow at Indiana University School of Medicine. I earned my PhD in medical clinical psychology from the University of Alabama at Birmingham after completing my clinical internship at Cincinnati Children’s Hospital. I believe research is more beneficial when both the questions are derived with the help of those with lived experience and the results are given back to them. I have found a variety of ways to do this, by presenting my work at patient- and family-based conferences, engaging with teens at summer camp, and using social media (follow me on Twitter @lynch_phd) to disseminate evidence-based recommendations and research findings. My position in the PRF Virtual Correspondents Program will help me reach an even wider audience! My emerging program of research seeks to better understand the protective resilience processes of youth experiencing chronic pain. Two important factors for youth with chronic pain include social support and engaging in healthy behaviors (e.g., sleep and physical activity). Through both qualitative and quantitative methods, I hope to understand how adolescents and young adults with chronic pain perceive their friendships and their common barriers to developing a supportive network. Additionally, I continue to conduct research evaluating health behaviors in youth with chronic pain in order to determine how to help pediatric patients capitalize on these lifestyle-based coping strategies. I am extremely excited to have been selected as a Correspondent for the Pain Research Forum. As a psychologist, I believe that communication of scientific research and evidence-based care is both an honor and an obligation. I will work to display my passion for pediatric pain over the coming weeks in order to foster scientific curiosity and show the resilience of our pediatric patients. Mary Lynch, PhD, postdoctoral fellow, Indiana University School of Medicine, US. A Binocular View of Pain: From Patient to Researcher Hi, PRF Readers! I’m Prab Ajrawat, an MSc candidate with the Department of Orthopaedic Surgery as well as the Department of Anesthesiology and Pain Medicine at the University of Toronto. Before getting into my research, I wanted to give readers some insight on my voyage into pain research and science communication. My journey began as a competitive athlete where I sustained an injury that prevented me from playing sports, particularly basketball. This transition from a competitive athlete to a chronic pain patient allowed for self-discovery in pain medicine and solidified my passion for pain research. Growing up, I’ve always been that individual who endlessly asks “why” with the intention of trying to find the end of the rope or the source of the issue. During my experience as a young patient, I often struggled to understand the scientific jargon that explained treatments or upcoming research. This duality of my patient experience along with my natural curiosity for things directed me to pain research, with an aim to explore novel therapies for chronic pain while learning how to effectively communicate research to other patients. Lately, my research has been focusing on using medical cannabis for alleviating chronic pain and exploring how medical cannabis modulates the immune system to lessen the symptoms of chronic pain. You might be thinking, “Well, why medical cannabis?” Frankly, I chose this area of research for two reasons. First, medical cannabis is portrayed by the media as a “cure-all” treatment, and I wanted to help clear the speculation and skepticism around using medical cannabis and accurately show its appropriate uses for certain patients and pain conditions. Second, with the recent legalization of cannabis in Canada, I thought it would be quite interesting to get involved in this emerging research field (and something that is outside my comfort zone), and see what all the fuss is about. I’m far from being an expert on medical cannabis, but that’s the beauty of doing research – as you start to learn a little, it feeds into your curiosity, which in turn creates questions that most of the time lack answers but spark investigations. I’m excited and grateful to have the opportunity to use the PRF platform as a PRF Virtual Correspondent to once again step outside my comfort zone and learn to communicate pain research to a range of audiences all over the world. Prab Ajrawat, MSc candidate, University of Toronto, Canada. When One Door Slams Shut, Break Another One Down Hello, PRF readers. I’m Sherelle Casey, and I have almost finished my PhD studies at the University of Sydney (I’m in the limbo period between thesis submission and officially being awarded the degree). As an 18-year-old first-year veterinary student, I thought I had my entire career planned out – I was going to work as a GP veterinarian for five years or so before devoting myself to studying and sitting the exams to become an internal medicine specialist. So how exactly does a veterinarian end up doing a basic science PhD aimed at informing human clinical pain trials? The short answer: pain. The long answer is that I had knee surgery in the middle of my vet degree and have since had stabbing pains in my knee(s) (diagnosed as complex regional pain syndrome or CRPS). I learned the hard way in my final year that being a vet is a lot more physical than it seems (standing, lifting, bending, etc.) and spent the year in agony. Thus, the door closed on my veterinary career. At that stage, I was angry at the failure of doctors and medicine to fix me, so I set about finding a pain researcher to take me on as a PhD student with the (somewhat naïve) goal of fixing myself. My search led me to Professor Christopher Vaughan, and I began my PhD journey. Through my PhD studies, I grew to love research (but not hurting mice). I submitted my PhD thesis (in which I examined the effects of the two major constituents of cannabis, THC and CBD, for the treatment of neuropathic pain) in June this year. I am now extremely interested in the therapeutic potential of cannabis (and am a firm believer that it does have potential, despite the problems in human trials). To this end, I am now going back towards my veterinary roots and working with a company that is doing clinical cannabis trials in dogs with pain and inflammatory conditions. My career goal is to make a positive difference in the lives of the many thousands of dogs with these conditions through being involved in the development of safe and effective medicinal cannabis products. Through both my research and my experiences as a pain patient, I have also developed a passion for science communication, and am very excited to have the opportunity to further develop these skills through the PRF Correspondents Program. It is of vital importance that researchers and healthcare professionals provide information in a format that can be understood by the patient population and their families (whether the patients have two legs or four). Understanding research gives people knowledge of what treatments are currently available, and what new treatments might be coming up. This knowledge empowers them to have a say in their treatment and, for the many people for whom current treatments have failed, provides hope that maybe one day there will be something that will work. Sherelle Casey, PhD student, University of Sydney, Australia Let's Talk Prevention – of Chronic Pain Chronic pain prevention is happening every day, but you may not notice it when it does occur, and research has not fully revealed how it works. Can you remember a time when you nearly twisted your ankle –or had another scary close call – but you didn't end up injured? It happened to me last week on a trail run – that familiar twinge of pain, the rush of adrenaline, a thought like, “Wow, that was close.” And then quickly, I moved on, because, well, it didn't happen. Or if you have twisted your ankle, you know that after weeks to months of healing, the next time you have another close call, you experience that similar twinge of pain to alert you to potential tissue damage. Either of these acute pain experiences could trigger a pain response that leads to chronic pain instead. Yet both of these are examples of successful chronic pain “prevention.” In both cases, pain information was processed in a way that was helpful for protecting tissue and resolved once tissue healed. Yet we still have a limited understanding of why it works that way sometimes, and for some people, but not always. When chronic pain “prevention” does not happen, the alerting signal for potential danger starts to malfunction. To illustrate this, imagine now instead that you softly brush your finger across your arm, and your brain insists that your arm is being lit on fire. How can we prevent the brain from making such inaccurate assumptions about the threat of injury, while at the same time ensuring that real threat continues to be processed to help us to avoid real injury? I am utterly fascinated by the idea that we can, and do, prevent chronic pain on a fairly regular basis, yet we understand very little of how that process naturally occurs. I am Wendy Gaultney, a postdoctoral fellow at Oregon Health & Science University. In our research lab, we study children and adolescents who are at heightened risk of developing chronic pain and observe them over time to identify risk and resilience factors for those who develop chronic pain. As you can probably tell, I am especially interested in the burgeoning field of chronic pain prevention. I am deeply grateful to all who recognized the value of providing this PRF Virtual Correspondents program, and to the research community that consistently inspires me to be curious and to learn how to communicate our questions, and hopefully, someday, to communicate our answers, to those who most need them. I am looking forward to the coming weeks of conversations with so many of you! Wendy Gaultney, PhD, postdoctoral fellow, Oregon Health & Science University, US. Hello, PRF readers! I am Y V Raghava Neelapala, an academic faculty member in the Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India. I have been living in the serene town of Manipal in South India for the past nine years and completed my postgraduate studies in orthopedic physiotherapy in the year 2013. Currently, I am looking for opportunities for doctoral studies in the pain sciences. I wish I could share more details about my future research avenues, but these have been delayed due to the COVID pandemic. I am very excited to be part of the PRF Virtual Correspondents Program this year, and in the coming weeks I would like to share some of the clinical and research challenges and (hopefully some solutions) encountered by physiotherapists in developing countries (India, in my case) when dealing with chronic pain. Since my internship days, I sought answers to the question of why some people with chronic pain get better with treatment while others do not recover at all. Also, I noticed that in some patients everything works, and in others nothing works. Studying the pain literature in my postgraduate days, I realized that pain is an experience unique to each person and at the same time caused by multiple factors. My clinical experience has taught me that a set of factors underlies the pain experience in each person with chronic pain, and the healthcare professional’s work lies in identifying and managing these unique and multiple factors accordingly. So that is all for now. I will see you again soon, with a mixture of mostly traditional content but some unorthodox content as well. Y V Raghava Neelapala, PT, MPT, assistant professor, Manipal College of Health Professions, Manipal Academy of Higher Education, India.