The American actress Danica McKellar, best known for her role as Winnie Cooper in the hit television series The Wonder Years, once said: “My hope is to go fully natural during childbirth – no epidural, no interventions. Wish me luck.”
A new study has found that luck may indeed have something to do with the experience of pain during childbirth –in this case, genetic luck. New work led by a collaboration at the University of Cambridge, UK, has identified a subset of women who have reduced pain during labor because of a genetic variant in KCNG4, a gene that encodes the voltage-gated potassium channel Kv6.4. Furthermore, laboratory experiments showed that mouse nociceptive neurons expressing this Kv6.4 variant had reduced electrical excitability, possibly underlying the reduced labor pain in these women.
“This paper is a beautiful example of how interdisciplinary research can reveal something so fundamental as human labor pain,” said Rajesh Khanna, University of Arizona, Tucson, US, who was not part of the study. “It is a landmark paper in its ability to show how a marriage of different fields – clinical, genetic, physiological, molecular – can come together to find a new gene mutation that explains a type of pain resilience in childbirth,” Khanna told PRF.
The paper was published July 21, 2020, in Cell Reports.
Resilience to labor pain
Geoffrey Woods, one of four senior authors including Ewan St. John Smith, Frank Reimann, and David Menon, explained how the collaboration came together to find new genetic mutations that underlie common variance in the pain experience.
“Patients with pain insensitivity are very important to study pain mechanisms in humans, but because they are extremely rare, we wanted to look at more common variances of pain. We aimed to investigate if there were genetic predispositions that made people less likely to experience pain in common circumstances,” Woods told PRF.
They chose to look for healthy women who did not require analgesia during full-term delivery of their firstborn child.
“From local health records, we estimated that about 1% of those women in the UK manage normal delivery without asking for Entonox [an inhaled gas used for analgesia] or epidurals,” according to Michael Lee, one of four co-first authors on the study along with Michael Nahorski, James Hockley, and Van Lu.
His team managed to identify 189 participants for the genetic study. The group also examined whether experimental pain thresholds were increased in these women.
“We focused on somatic pain thresholds because we didn’t have a visceral pain stimulus that mimics labor. I know colleagues who would happily put balloons up orifices to stimulate the viscera in animal models, but it is not something I wish to do in humans,” Lee said humorously.
Strikingly, the results showed that women who did not require analgesics during labor had elevated heat, cold, and mechanical pain thresholds, with a particularly striking correlation between cuff pain pressure thresholds and reduced labor pain. This suggests that this cohort of women had higher pain thresholds in general.
“It’s important that these women are healthy, and performed the same as controls on cognitive and psychometric testing. The genetic mutation discovered seems to have analgesic effects only,” Lee added.
A genetic hit in KCNG4
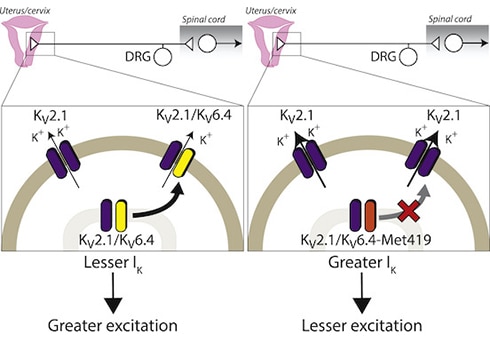
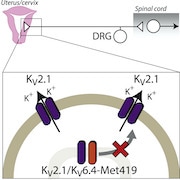
The authors next analyzed DNA sequences from the 189 women who did not require analgesia during labor, in the hope of identifying novel genes involved in pain. Popping out of the sequencing results was an overrepresented single nucleotide polymorphism (SNP) in the gene KCNG4, which encodes the voltage-gated potassium channel subunit Kv6.4, in four patients. In this case, the SNP causes a heterozygous missense mutation in KCNG4
“Amazingly, of all the places the mutation could be in KCNG4, it happens to be in a region of the channel that is a potassium selectivity filter,” Khanna commented. On its own, Kv6.4 is an electrically silent potassium channel subunit, but it influences potassium currents when it forms ion channel complexes called heterotetramers with Kv2.1. This led the researchers to hypothesize that a loss-of-function Kv6.4 mutation would change the ability of Kv6.4 to bind to Kv2.1 and would therefore dampen the excitability of nociceptors. To test this, the researchers turned to in vitro experiments using human embryonic kidney 293 (HEK293) cells. They overexpressed wild-type Kv6.4 or the mutant Kv6.4 in the HEK cells and performed electrophysiological experiments on single cells. Importantly, because Kv6.4 does not produce currents on its own, the authors co-expressed Kv2.1 and measured the effect of the mutant Kv6.4 on Kv2.1-mediated potassium currents. As expected, wild-type Kv6.4 shifted the electrical properties of Kv2.1 such that it was inactivated at more negative voltages. However, this effect was lost with mutant Kv6.4, showing that this mutation prevents Kv6.4 from modulating Kv2.1 and therefore from altering cell excitability. Moreover, immunofluorescence staining revealed that mutant Kv6.4 was unable to reach the cell membrane from the cytoplasm and form heterotetramers with Kv2.1, which neatly explained why Kv2.1 was left unmodulated in homomeric channels at the cell membrane. To the mouse! But what do all of these findings mean in terms of nociception and labor pain? The cell line data suggested that the SNP found in the four women who did not require analgesia during labor caused a loss of function in Kv6.4, which in turn failed to modulate Kv2.1. The authors hypothesized that if this were to occur in nociceptors, it could reduce the electrical excitability of such cells. To delve deeper into the mechanism, the collaboration grew to include Ewan St. John Smith. “We had two questions to ask using a mouse model: First, is Kv6.4 present in uterine dorsal root ganglia [DRG] nociceptors? And second, if you drive this Kv6.4 mutation in sensory neurons, does it change nociceptor excitability?” Smith said. To answer the first question, the researchers used a dye called fast blue to label DRG sensory neurons that project to the mouse uterus and then dissected them out and performed single cell quantitative reverse transcription polymerase chain reaction (qRT-PCR) testing to measure the expression of Kcng4 mRNA. Kcng4 mRNA was indeed found in subsets of thoracolumbar and lumbosacral DRG neurons that projected to the uterus. Moreover, this expression overlapped with the nociceptive neuron markers Trpv1 and Scn10a (which encodes the Nav1.8 sodium channel), suggesting that the Kv6.4 channel is present in nociceptors. But would mutant Kv6.4 change nociceptor excitability? To answer this question, the authors performed electrophysiological recordings from isolated DRG neurons in which they had overexpressed the mutant Kv6.4 they had identified in their earlier experiments. They found that these neurons had higher action potential thresholds and were therefore less excitable than those transfected with wild-type Kv6.4. “With the mutant Kv6.4, what you end up with is a lot more homomeric Kv2.1 at the plasma membrane. Because of Kv2.1’s inactivation properties, this means you have a lot more potassium flux around the resting membrane potential, meaning you need more input to counteract it and drive the action potential firing. Thus it seems that sensory neurons that express the mutant Kv6.4 need more nociceptive input to drive them,” Smith told PRF. A dominant negative effect Because the KCNG4 SNP was heterozygous in the patient cohort, the authors next tested whether this effect was dominant negative, in other words, whether the single mutated KCNG4 allele can override the wild-type KCNG4 allele and drive the cell excitability phenotype. So in one final experiment, the authors turned back to the HEK cell line to co-transfect either the mutant Kv6.4 with wild-type Kv6.4 to mimic a heterozygous mutation, or only mutant Kv6.4 to mimic a homozygous mutation. The results confirmed that Kv6.4 indeed acts in a dominant negative way to reduce labor pain, as both membrane localization of Kv6.4 and electrical excitability of HEK cells was perturbed in both the heterozygous and homozygous situations. Overall, Smith stressed that the findings from the new study could be a starting point to investigate and treat visceral pain. “There wasn’t anything out there on how Kv6.4 might modulate pain sensation, let alone visceral pain. So maybe we could target Kv6.4 for other types of pain, particularly visceral pain, which is poorly treated compared to other types of pain. It’s another body of work to be done, but the starting point looks interesting,” Smith said. Khanna agreed, and highlighted the wider implications of the study. “It would be interesting to see if these women [with the KCNG4 SNP] suffer as much from other visceral pain conditions like irritable bowel syndrome, and especially whether this is more present in women than men.” If so, perhaps the luck of genetics would provide pain relief even well beyond the delivery room…. Fred Schwaller, PhD, is a freelance science writer based in Germany.