Major efforts are underway to develop new opioid drugs that relieve pain without dangerous adverse effects. One strategy relies on biased agonism—a term that describes preferential activation of one downstream signaling pathway over another upon binding of an agonist to a receptor. In the case of mu-opioid receptor (MOR) agonists, researchers are seeking compounds that activate pathways responsible for analgesia while avoiding pathways that mediate negative outcomes like respiratory depression and tolerance.
Now, a peptide isolated from a fungus found in an extremely remote place in the world seems to activate MOR with biased agonism, favoring G protein signaling, thought to underlie analgesia, while avoiding beta-arrestin signaling, which regulates multiple adverse effects of opioids, according to previous studies. The peptide contained just four amino acids in a never-before-seen structure that fit perfectly into the MOR binding pocket.
“The study is very comprehensive and elegant,” wrote Anne Murphy, who studies opioid signaling at Georgia State University, Atlanta, US, but was not involved in the current work, in an email to PRF. “The discovery of a novel G protein-biased MOR agonist holds great clinical promise and will undoubtedly pave the way for the development of new pain therapeutics that minimize both the negative side effects and risks associated with currently available opiates.”
The new work from Macdonald Christie, University of Sydney, Australia; Paul Alewood and Robert Capon, University of Queensland, Brisbane, Australia; and colleagues was published October 29 in PNAS.
Left, right, left, right
Opioid effects, ranging from pain relief to respiratory depression and tolerance, arise from activation of the MOR, a G protein-coupled receptor (GPCR). Researchers long assumed that agonists either switched on G protein signaling or they did not. But they have come to recognize that this idea of an “on/off” switch was an oversimplification.
While MORs associate with and signal through different types of G proteins in various cells, they also bind to other types of proteins, initiating distinct signaling pathways with different consequences. The pain-relieving effects of opioids are thought to arise from G protein signaling, whereas recruitment of the regulatory protein beta-arrestin has been associated with a number of negative side effects (see related PRF coverage here and here).
For the current study, the researchers found the new peptides by screening extracts from the fungus Penicillium sp. MST-MF667, which they discovered in a pristine Tasmanian estuary. Three tetrapeptides (peptides made of only four amino acids) called bilaids bore an unusual chirality, or molecular arrangement.
Whereas “99.999 percent of amino acids in the world are left-handed,” said Christie, these peptides contained right-handed, mirror-image amino acids. “This structure had never been seen before,” according to Christie, describing the tetrapeptides’ alternating left, right, left, right (LDLD) chirality.
As unusual as the structure was, co-first author Zoltan Dekan, University of Queensland, Australia, noticed that the peptides bore an uncanny resemblance to mammalian endogenous opioid peptides. (Additional co-first authors are Setareh Sianati and Arsalan Yousuf, University of Sydney, Australia, and Katy Sutcliffe, University of Bristol, UK.)
Indeed, the bilaids bound the human MOR with modest affinity, Christie told PRF. “Then we made some modifications, in ways well understood to increase potency, and got a 1,000-fold increase in potency and selectivity” with a compound they called bilorphin.
“Completely the opposite”
Christie and colleagues then tested bilorphin’s functional activity by patch-clamping neurons from the rat locus coeruleus (LC). These neurons contain MORs (but not delta- or kappa-opioid receptors) and G protein-activated, inwardly rectifying potassium (GIRK) channels, a downstream target of MOR activation.
Bilorphin activated GIRK-mediated currents with higher potency than did morphine. To better quantify GIRK activation, the researchers then moved to a mouse cell line stably expressing mouse MOR. Bilorphin, morphine, and the endogenous opioid tetrapeptide endomorphin-2 all activated GIRK channels to a similar extent, and to a slightly lesser degree than did Met-enkephalin, also an endogenous opioid.
The researchers also compared bilorphin to another agonist that had caught the attention of pain researchers. Oliceridine is a MOR agonist developed by the biopharmaceutical company Trevena, and has undergone clinical trials for acute pain. Compared to morphine, this agent, which was the first biased agonist to be tested in people, was biased toward G protein signaling over beta-arrestin recruitment, in an assay measuring accumulation of cyclic AMP (cAMP), an effect of G protein signaling (DeWire et al., 2013). In the new study, oliceridine was less effective at engaging GIRK channels than were the other opioids, including bilorphin.
(Importantly, it should be noted that in clinical studies completed in 2017, oliceridine only avoided respiratory depression at the lowest tested dose, introducing uncertainty about the biased agonism approach toward developing new analgesics. One possibility is that the compound was not biased enough toward G protein signaling [see PRF related coverage]. Despite failure to win approval by the US Food and Drug Administration [FDA], Trevena recently announced plans to resubmit a new drug application for oliceridine in 2020, according to a recent press release.)
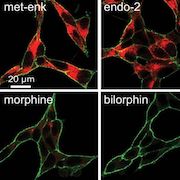
For the new study, the team next measured phosphorylation of the MOR C-terminus, recruitment of beta-arrestin, and receptor internalization—all thought to contribute to side effects such as tolerance and dependence—following treatment with bilorphin or morphine, in the mouse cell line. Bilorphin induced low levels of phosphorylation similar to morphine, but significantly less beta-arrestin recruitment, and only negligible receptor internalization comparable to that seen with oliceridine. This was in contrast to Met-enkephalin and endomorphin-2, which are biased toward beta-arrestin signaling and showed robust internalization.
“This was completely the opposite of all the known endorphins in terms of bias,” Christie said of bilorphin. “It was really efficacious at activating G proteins in vitro, but it didn’t signal to beta-arrestins at all. So we knew we had a very unusual and natural form—a molecule worth exploring.”
![Example images of MOPr [mu-opioid receptor] internalization 30 min after incubation with saturating concentrations of agonists. Dual staining was employed for quantification (membrane receptor in green and internalized receptor in red, colors enhanced uniformly for presentation purposes). Image and caption from Dekan et al. Proc Natl Acad Sci U S A. 2019 Oct 29;116(44):22353-22358. Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND).](https://www.iasp-pain.org/wp-content/uploads/2023/02/BilorphinInline290.jpg)
Different conformations
Biased signaling is thought to arise from distinct protein conformations formed and stabilized when a particular agonist binds to a receptor, which in turn encourages interactions with some downstream signaling proteins over others. To see how bilorphin affected MOR, a cell surface receptor with multiple transmembrane domains, the researchers used computer modeling to simulate molecular docking and molecular dynamics of bilorphin binding to the MOR, and compared it with MOR bound to endomorphin-2. Molecular docking and dynamics refer to how agonists bind and interact with static and dynamic (respectively) models of a protein structure.
According to the simulation, from the binding pocket, bilorphin extended to the extracellular side of MOR and interacted with transmembrane domains 1, 2, and 7, whereas endomorphin-2 interacted with the receptor’s extracellular loops. Further modeling revealed differences in the receptor’s conformation when bound by bilorphin compared to when bound by endomorphin-2, which the authors suggest might account for their opposite signaling biases.
The researchers next looked at the in vivo effects of their compound. Bilorphin was antinociceptive in the hotplate test in mice only after intrathecal but not intravenous or subcutaneous delivery. This indicated that it did not cross the blood-brain barrier (BBB).
To improve BBB permeability of bilorphin, Christie and colleagues developed an analog by glycosylating bilorphin near its C-terminus. When delivered systemically, this analog, called bilactorphin, provided analgesia similar to morphine that was blocked by the MOR antagonist naltrexone.
Bilactorphin also led to significantly more beta-arrestin recruitment and receptor internalization than did bilorphin in the mouse cell assay, demonstrating that it did not retain the same G protein-biased signaling. Nevertheless, Christie said the core tetrapeptide backbone of bilorphin can serve as a structural framework to design and build beneficially biased opioids. Murphy agrees.
“If the group is successful in making further modifications to a bilorphin analog, such that it’s BBB permeable while still maintaining G protein bias at MOR, it will be a strong contender as a next-gen analgesic,” Murphy wrote to PRF.
William Schmidt, a drug development expert with NorthStar Consulting, LLC, Davis, US, who was not involved in the new study and has no connection with Trevena, is still cautiously optimistic about the biased agonism approach.
“Is this the only way to get safer opioid analgesics, i.e., less respiratory depression, less constipation, lower addiction liability, and less opioid tolerance development? Maybe not. This is where we need more data, whether it’s from biased ligands, modified peptides, or compounds that bind to different splice variants of the mu-opioid receptor. Ultimately, I don’t care what the mechanism of action is as long as the new compounds demonstrate superior analgesia and lower side effect profiles in humans,” Schmidt wrote in an email to PRF.
Stephani Sutherland, PhD, is a neuroscientist and freelance journalist in Southern California. Follow her on Twitter @SutherlandPhD