The anterior cingulate cortex (ACC) has long been known as a key node in processing affective components of pain in animals and people. But little is known about the ACC neurons themselves or the circuits that connect them with other brain areas. Now, a new study probing how inputs to the ACC regulate pain-related aversion in mice reveals distinct microcircuitry with unexpected complexity.
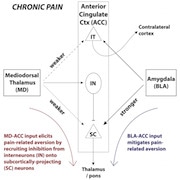
Because previous studies had shown that increased activity in the ACC was associated with the aversive component of chronic pain conditions, researchers assumed that neurons throughout the region might become hyperexcitable. But the new work shows that pain aversion actually depended on inputs from the mediodorsal thalamus (MD) to the ACC that resulted in decreased activity in a subpopulation of neurons. Activation of inputs from the basolateral amygdala (BLA), in contrast, increased activity in a different ACC neuronal population, which unexpectedly mitigated pain-related aversion.
The work is a collaboration between the laboratories of pain researcher Allan Basbaum and Vikaas Sohal, a psychiatrist who studies cortical circuitry, both at the University of California, San Francisco, US. The study was published June 5, 2019, in Neuron, along with an accompanying “Preview” by Nur Zeynep Gungor and Joshua Johansen, RIKEN Center for Brain Science, Wako-shi, Japan.
“This is a very interesting paper because it challenges the current view of what people are thinking” about the ACC, said Feng Wei, who studies the ACC at the University of Maryland, Baltimore, US, but was not involved in the current work. “They used optogenetics to dissect the circuit at the cellular level, and it looks like, in this cell-based analysis, that some neurons are not what we think.”
Specific input-output pathways control pain-related aversion
The ACC has been recognized for some time to be involved in the affective component of pain.
“Interestingly, the ACC was actually implicated first in humans where fMRI and early PET studies showed that the ACC lights up as a function of the unpleasantness of the pain experience,” said Basbaum. And some patients with intractable pain who received an ACC lesion found relief from suffering but still had the sensory perception of pain. “So there is plenty of reason to think the ACC is critical to the affective experience,” he said, but researchers have little understanding of the details of how.
“When you look at a patient fMRI, it can tell you the ACC is activated, but that’s it. Which layer of neurons is involved? Are the neurons excitatory or inhibitory? What kind of molecular mechanisms are involved? We don’t know,” said Wei.
To learn more, in the current study the researchers used two mouse models of chronic neuropathic pain: spared nerve injury (SNI) and chemotherapy-induced pain caused by the drug Taxol. In a conditioned place preference (CPP) paradigm, mice that received SNI or Taxol preferred the side of a chamber where they received a dose of gabapentin, which is used clinically for neuropathic pain, indicating the animals were experiencing pain that was relieved by the drug.
First author Karuna Meda and colleagues then focused on two sources of inputs to the ACC previously implicated in pain: the MD and the BLA. “The MD and other thalamic nuclei are the main transmitting station for nociceptive information coming from the spinal cord to the ACC,” Meda said. She led the collaborative project as a graduate student in the Basbaum and Sohal labs, and has since graduated.
To manipulate the inputs with optogenetics, Meda and colleagues injected mice with a virus to drive expression of the light-sensitive protein channelrhodopsin-2 (ChR2) in the MD, and then implanted an optic fiber above the ACC, where light would activate the MD input terminals. Three weeks later, mice received either Taxol or SNI, and were tested in the CPP paradigm seven to 10 days after injury. For CPP, mice received optogenetic stimulation on one side of a chamber. The next day, Meda measured how much time mice spent on either side relative to baseline before stimulation.
“We found that when we activated MD inputs, it actually seemed to make the pain worse, so animals avoided the side that was paired with activation,” Meda said. Control mice without neuropathic pain did not prefer one side over the other after MD-ACC stimulation.
In another experiment, mice expressed archaerhodopsin, a light-sensitive channel that inhibits neurons upon optic stimulation, in MD neurons. Mice with SNI as well as pain-free mice preferred the side of a chamber where they received optogenetic stimulation that inhibited MD inputs to the ACC, confirming that those inputs transmit an aversive signal.
Meda next investigated a second source of inputs to the ACC, the BLA. “The BLA is involved more generally with the processing of aversive stimuli; it’s an important part of that limbic circuit,” Meda said. “Activating MD inputs made pain worse, which goes along with the theory that driving input to the ACC makes pain worse. But when we activated BLA inputs, we found the opposite. Animals actually preferred contexts that were paired with that manipulation,” suggesting BLA inputs mitigated pain rather than exacerbated it.
“I appreciate this paper because they were not just looking at one pathway, but they’ve shown two input pathways, one from the mediodorsal thalamus and another from the amygdala, with different functions—like a yin and yang for a balance of ACC neuronal activity,” Wei said.
The balance between excitation and inhibition
Imaging studies have suggested that the ACC becomes hyperactive in chronic pain states. So the researchers were surprised when electrophysiological experiments in slices from the rostral ACC showed that ACC responses to MD inputs were weakened, rather than strengthened, in SNI or Taxol-treated animals compared to uninjured mice.
“We know the ACC and the MD turn on in pain,” Sohal said. “So if I stimulate input from the MD to the ACC, and it makes pain worse, the reason probably is because the connections become stronger and more excitatory. And we found mostly the opposite of that.”
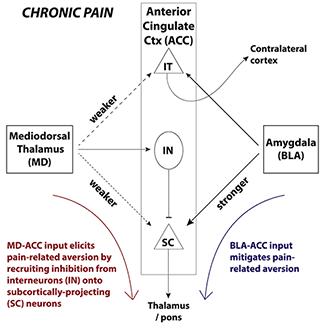
To take a closer look at these electrophysiological properties, the researchers differentiated between two distinct neuronal types within layer V of the ACC that receive MD inputs: subcortically projecting (SC) neurons, and intratelencephalic (IT) neurons that project to contralateral cortex. Both cell types from animals with pain were hyperexcitable relative to those from pain-free mice.
To examine the effects of MD inputs specifically, the researchers activated them optogenetically and measured excitatory postsynaptic currents (EPSCs) from SC and IT neurons. Peak currents and probability of spiking were significantly reduced in SC neurons from animals with SNI or Taxol compared to pain-free controls. Further electrophysiological experiments pointed to a postsynaptic mechanism behind the weakened responses.
The ratio of excitatory to inhibitory (E/I) postsynaptic currents measured in response to optic stimulation of MD inputs indicated that animals with pain exhibited a shift toward inhibitory responses compared to pain-free mice. In uninjured mice, activating MD inputs significantly increased SC neuron spiking, but in neurons from SNI or Taxol mice, spiking did not increase or even decreased.
In contrast, optogenetic activation of BLA inputs in slices of rostral ACC led to significantly larger EPSCs in SC neurons from injured mice than in controls. IT neuron responses to BLA inputs did not significantly differ in the pain conditions.
“It’s true that MD input makes pain worse, but it’s because it becomes more inhibitory and weaker,” said Sohal. “And it’s true that BLA inputs become stronger, but that actually tends to mitigate, not exacerbate pain. It’s really exciting.”
Finally, to test whether direct inhibition of SC neurons exacerbated pain, Meda used a retrograde transport viral strategy to infect SC neurons with archaerhodopsin. Injured but not pain-free mice avoided the place where they received optogenetic inhibition of SC neurons, in a CPP paradigm.
“This was the solidifying experiment because it showed when we shut off those neurons, it replicated what we saw with MD activation. It helped us prove our model, essentially, which is that reducing the activity of SC neurons seems to be exacerbating the pain.” Conversely, mice preferred the place where they received optogenetic activation of SC neurons.
In all animals, MD inputs preferentially activated SC neurons, whereas BLA inputs activated SC and IT neurons equally.
“Understanding this microcircuitry shows how the ACC actually works,” Wei said, “and how it is involved in the affective component of chronic pain.”
Heterogeneity abounds
The role of the ACC has been investigated in pain for decades, Basbaum said, “but it’s basically been seen as this big blob of tissue. Little was known about how circuits within the ACC influence pain experience, or whether there was any neuronal heterogeneity.”
It’s clear from the new study that ACC neurons are indeed heterogeneous, and that microcircuits connecting the ACC and MD may have very different effects. “It matters not only where information is coming from, but where it’s ending up, at which neuron type,” Meda said.
Optogenetic inhibition of IT neurons did not lead to place preference or avoidance in mice with or without pain. In contrast, Meda said, “optogenetically reducing the activity of SC neurons seems to exacerbate the pain. That highlights the importance of neuronal heterogeneity; when we shut off IT neurons, it did not have the same effects as inhibiting SC neurons. The next questions are about where the information goes next, who are the SC neurons talking to—or not—and what are the downstream mechanisms?”
While the researchers have identified a new microcircuit, it is just one small part of the very complicated ACC circuitry and the even broader pain circuitry throughout the brain. “We have discovered this one strand of this circuit, and we are just going to keep following it to see where it goes,” Sohal said.
The team hopes that better understanding of the nature of ACC neuronal subpopulations and how they connect to other brain regions might help researchers better target ACC mechanisms—either pharmacologically or through deep brain stimulation, for example—to alleviate the suffering of pain.
Stephani Sutherland, PhD, is a neuroscientist and freelance journalist in Southern California. Follow her on Twitter @SutherlandPhD